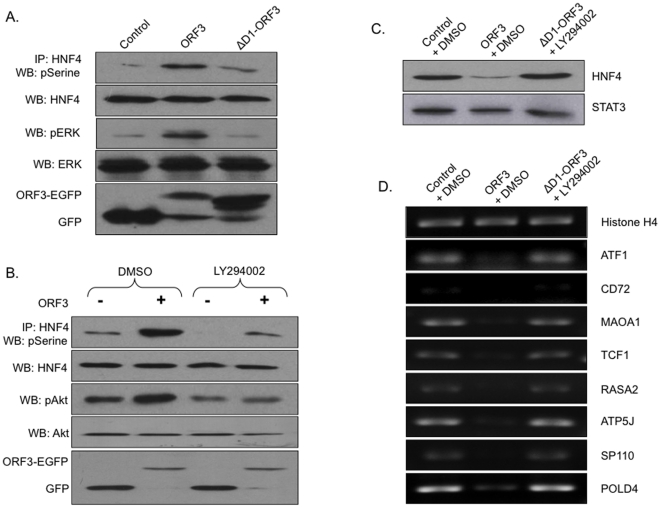
Figure 5. The ORF3 protein modulates HNF4 phosphorylation.
(A) Huh7 cells were transfected to express EGFP (control) or the ORF3-EGFP or ΔD1-ORF3-EGFP fusion protein, and cells were harvested 48 hr post-infection. Cell lysates containing equal amounts of total protein were immunoprecipitated with anti-HNF4 and then western blotted with anti-pSerine antibody. Normalized lysates were western blotted with anti-HNF4 and anti-GFP antibodies for loading and expression controls, respectively. Normalized lysates were also western blotted with anti-pERK and anti-ERK to show the effect of pORF3 on ERK activation. (B) Huh7 cells were infected with ORF3 expressing (+) and control (−) recombinant adenoviruses. At 36 hr post infection, the cells were treated with either 50 µM LY294002 or an equal volume of DMSO for 12 hr. Cells were harvested 48 hr post-infection and lysates containing equal amounts of total protein were immunoprecipitated with anti-HNF4 and followed by western blotting with anti-pSerine antibody. Normalized lysates were western blotted with anti-HNF4 and anti-GFP antibodies for loading and expression controls, respectively. Normalized lysates were also western blotted with anti-pAkt and anti-Akt to show the effect of pORF3 on Akt activation. (C) Huh7 cells were transfected to express EGFP (control) or the ORF3-EGFP or ΔD1-ORF3-EGFP fusion protein. At 36 hr post-transfection, the cells transfected with ΔD1-ORF3-EGFP expressing plasmid were treated with 50 µM LY294002 and others were treated with an equal volume of DMSO for 12 hr. Nuclear lysates were prepared and western blotted with anti-HNF4. STAT3 served as a loading control. (D) Huh7 cells were transfected and treated as described in (C). RT-PCR analysis was performed as mentioned earlier. Histone H4 served as a loading control for RNA.