Abstract
The mechanisms underlying neuropathic pain induction are very complex but might involve abnormal spontaneous activity in the sensory dorsal root ganglion (DRG). Voltage-gated sodium channels in the DRG are essential for the genesis of abnormal spontaneous neuronal activity. In the present study, we examined the changes in expression of the voltage-gated sodium channel Nav1.1 in the DRG after peripheral nerve injury. Western blot analysis showed that the level of Nav1.1 protein in the ipsilateral L5 DRG was significantly increased on days 3 and 7 after fifth lumbar spinal nerve ligation. Immunohistochemical study further confirmed a marked increase in the percentage of Nav1.1-positive cells in the ipsilateral DRG on day 3 after fifth lumbar spinal nerve ligation. Similarly, on day 7 after sciatic nerve axotomy, the amount of Nav1.1 protein and the percentage of Nav1.1-positive cells in the ipsilateral L5 DRG were also significantly increased. Our results suggest that an early increase in DRG Nav1.1 expression after peripheral nerve injury might be involved in the induction of neuropathic pain.
Keywords: Nav1.1, sodium channel, neuropathic pain, dorsal root ganglion, rat
Current treatments for neuropathic pain are often inadequate, ineffective, or produce potentially severe adverse effects because the mechanisms underlying neuropathic pain are far more complicated than assumed (Costigan et al., 2009). It is generally believed that peripheral and central sensitization after nerve injury contributes to the development and maintenance of neuropathic pain (Basbaum et al., 2009). Abnormal spontaneous activity that arises in neuromas at the sites of nerve injury and in the injured and adjacent uninjured dorsal root ganglion (DRG) neuronal cell bodies is considered to play a leading role in the genesis of neuropathic pain (Campbell and Meyer, 2006). Voltage-gated sodium channels, which are essential for generation and conduction of electrical impulses in excitable cells, may be key players in production of abnormal spontaneous activity in the DRG (Dib-Hajj et al., 2010). Sodium channels consist of a large α-subunit and one or more auxiliary β-subunits. Nine α-subunits (Nav1.1–Nav1.9, also referred to as channels) and four β-subunits have been identified in mammals. However, whether and how sodium channels are involved in the induction of neuropathic pain is still unknown or controversial (Dib-Hajj et al., 2010; Mo et al., 2011; Wang et al., 2011).
Nav1.1 is expressed widely in both peripheral and central nervous systems. Mutations of the gene SCN1A (which encodes Nav1.1) in the nervous system have been associated with human inherited epileptic syndromes and familial hemiplegic migraine, suggesting that Nav1.1 might participate in the genesis of migraine (Dichgans et al., 2005; Vahedi et al., 2009). In the DRG, in situ hybridization histochemistry revealed that Nav1.1 mRNA is expressed in both large- and small-diameter sensory neurons (Fukuoka et al., 2008), but whether DRG Nav1.1 is involved in neuropathic pain is unknown. Here, using Western blot analysis and an immunohistochemical approach, we examined changes in expression of Nav1.1 protein in the DRG after fifth spinal nerve ligation (SNL) and sciatic nerve axotomy.
MATERIALS AND METHODS
Animals and surgery
All experimental procedures received prior approval from the Animal Care and Use Committee at the Johns Hopkins University. Animal procedures were consistent with the ethical guidelines of the National Institutes of Health and the International Association for the Study of Pain and the ethical guidelines to investigate experimental pain in conscious animals (Zimmermann, 1983). Efforts were made to minimize animal suffering and to reduce the number of animals used.
SNL was carried out according to our previous protocols (Zhang et al., 2003; Guan et al., 2009; Singh et al., 2009). Briefly, adult male Sprague-Dawley rats (180–220 g) were anesthetized with isoflurane. Then the left lumbar (L) 6 transverse process was removed to expose the L4 and L5 spinal nerves. The L5 spinal nerve was carefully isolated and tightly ligated with 6-0 silk thread and then transected distal to the site of ligature. The surgical procedure for the sham group was identical to that of the SNL group, except that spinal nerves were not ligated or transected. For the sciatic nerve axotomy, rats were anesthetized with isoflurane, and the left sciatic nerve was exposed proximal to the pyriform ligament and transected; a 5-mm-length of the nerve was removed to prevent regeneration.
Behavioral testing
Behavioral testing was carried out as described previously (Guan et al., 2009; Singh et al., 2009). Briefly, mechanical paw withdrawal thresholds (PWTs) were measured with the up–down testing paradigm 1 day prior to surgery and on days 3 and 7 after SNL or sham surgery. Von Frey hairs in log increments of force (0.38, 0.57, 1.23, 1.83, 3.66, 5.93, 9.13, 13.1 g) were applied for a duration of 4–6 s to the region between the foot pads in the plantar aspect of the hind paw. The 1.83-g stimulus was applied first. If a positive response occurred, the next smaller von Frey hair was used; if a negative response was observed, the next higher force was used. The test was continued until: (1) the responses to five stimuli were assessed after the first crossing of the withdrawal threshold or (2) the upper/lower end of the von Frey hair set was reached before a positive/negative response had been obtained. Abrupt paw withdrawal, licking, and shaking were regarded as positive responses.
Western blotting
Animals were sacrificed, and bilateral L5 DRGs were rapidly collected. To obtain enough protein, the unilateral DRGs from 3 rats were pooled together. Experiments were repeated three times (N = 9/group). Briefly, the tissues were homogenized in chilled lysis buffer (50 mM Tris, 1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA, 1 µM leupeptin). The crude homogenate was centrifuged at 4°C for 15 min at 1,000g. The supernatant was collected, and the pellet (nuclei and debris fraction) discarded. After protein concentration was measured, the samples were heated at 99°C for 5 min and loaded onto a Bio-Rad Criterion Tris-HCl gel (Bio-Rad Laboratories, Hercules, CA, USA). The proteins were then electrophoretically transferred onto a polyvinylidene difluoride membrane (Immobilon-P, Millipore, Billerica, MA, USA). Because molecular weights of Na1.1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, a loading control) are significantly different (Fig. 1), the blotting membrane was cut into two parts. After being blocked in Tris-buffered saline containing 0.02% Tween-20 and 3% non-fat milk for 1 hr, two parts of the membranes were incubated with primary rabbit anti-Nav1.1 (1:200; Millipore) and goat anti-GAPDH (1:1,000; Santa-Cruz Biotechnology, Santa Cruz, CA, USA), respectively, overnight under gentle agitation. The proteins were detected by horseradish peroxidase-conjugated anti-rabbit or anti-goat secondary antibodies and visualized by chemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, NJ) and exposure to film. The intensity of blots was quantified with densitometry by investigators blind to the treatment groups. The same size of the rectangle was drawn around or near (for background) each band. The density of each band was quantified with the corresponding background subtraction. The blot density from the ipsilateral side on day 0 after SNL or axotomy was set as 100%. The relative density values from the contralateral side on day 0 after SNL or axotomy and from the ipsilateral and contralateral sides on days 3 and 7 after SNL or axotomy were determined by dividing the optical density values from these groups by the value from the contralateral side in the sham group after they were normalized to the corresponding GAPDH.
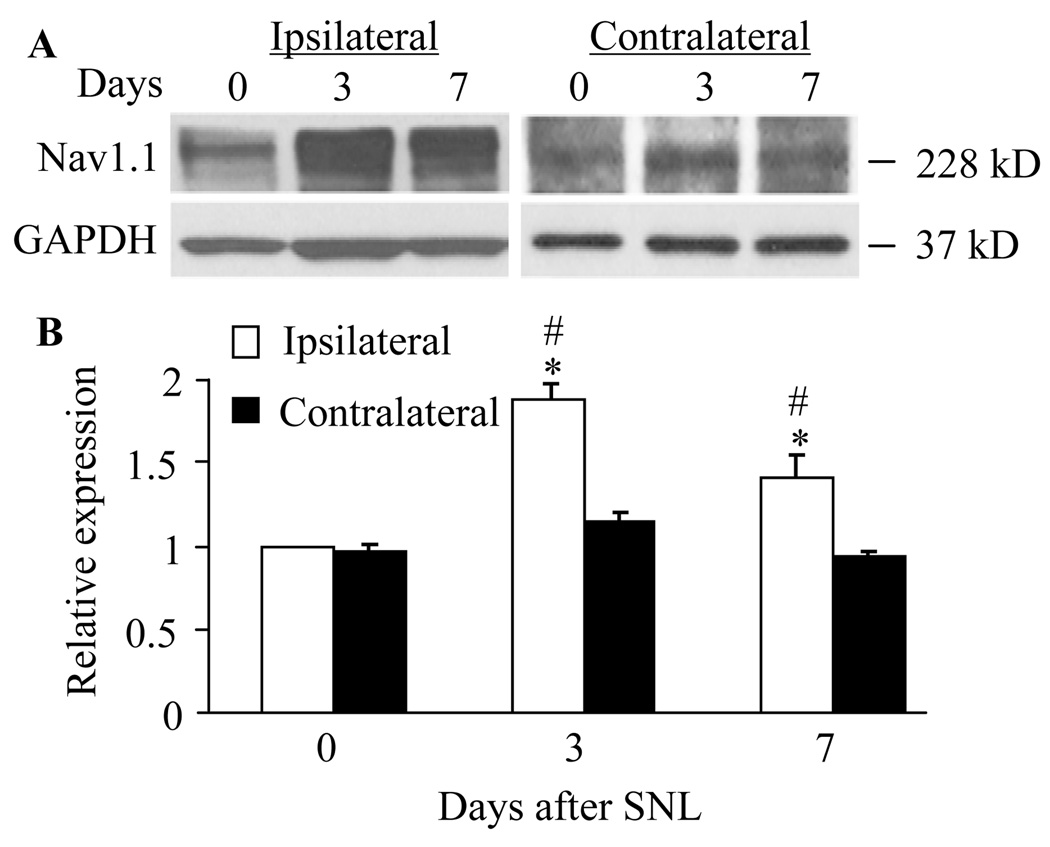
Fig. 1.
Spinal nerve ligation increased the level of Nav1.1 protein in the L5 dorsal root ganglia (DRG). A: Representative Western blots showing expression of Nav1.1 in the DRG of naïve rats (day 0) and of rats on days 3 and 7 after SNL. B: Statistical summary of the densitometric analysis showing the expression of Nav1.1 relative to that of ipsilateral DRG from naïve rats after normalization to the corresponding glyceraldehyde 3-phosphate dehydrogenase density. * P < 0.05 vs the corresponding naive group. # P < 0.05 vs the corresponding contralateral side.
Immunohistochemistry
The rats were anesthetized and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3). Bilateral L5 DRGs were collected, post-fixed in the same fixative solution for 2 hr, cryoprotected for 24 hr at 4°C in 0.1 M phosphate buffer containing 30% sucrose, and frozen-sectioned at 20 µm. After being blocked in 0.01 M phosphate-buffered saline containing 0.3% Triton X-100 and 10% goat serum for 1 hr at room temperature, the sections were incubated with primary rabbit anti-Nav1.1 (1:100; Millipore) overnight at 4°C and then with goat anti-rabbit IgG conjugated with Cy3 (1:300; Jackson ImmunoResearch, West Grove, PA, USA) for 1 hr at room temperature. Images were examined under a Nikon TE2000E fluorescence microscope (Nikon Co., Japan) and captured with a CCD spot camera. Nav1.1-positive and -negative DRG cells with nuclei were counted by investigators blind to the treatment groups. The mean percentage of Nav1.1-positive cells for each group was calculated from at least 12 sections from 3 rats. For size distribution analysis of Nav1.1-positive cells, approximately 36 sections from three rats (12–14 sections/rat) were randomly selected. Cell profiles were outlined and cell area was calculated by using the imaging software Image-Pro Plus (Media Cybernetics, Silver Spring, MD). The specificity and selectivity of the immunostaining were examined in parallel by omission of the primary specific antibodies.
Statistical analysis
Results from the behavioral study, Western blot analysis, and immunohistochemistry were statistically analyzed with a one-way or two-way analysis of variance (ANOVA). Data are presented as means ± SEM. When ANOVA showed significant difference, pair-wise comparisons between means were tested by the post hoc Tukey method. Significance was set at P < 0.05. The statistical software package SigmaStat (Systat, San Jose, CA, USA) was used to perform all statistical analysis.
RESULTS
To ensure that all SNL rats used for Western blot and immunohistochemistry analysis showed pain hypersensitivity, we measured PWTs in response to mechanical stimuli. Consistent with our previously published data (Guan et al., 2009; Singh et al., 2009), SNL induced mechanical hypersensitivity, as indicated by a significant decrease from baseline in ipsilateral (but not contralateral) PWT on days 3 and 7 post-surgery. Rats that did not show a significant decrease in PWT post-SNL were excluded from Western blot and immunohistochemistry analysis. As expected, sham surgery did not cause mechanical hypersensitivity on either the ipsilateral or contralateral side. Consistent with our previous work (Guan et al., 2009), after axotomy, the rats displayed no responses to mechanical stimuli on the ipsilateral side; however basal responses to stimuli of the contralateral hind paw were intact during the observation period.
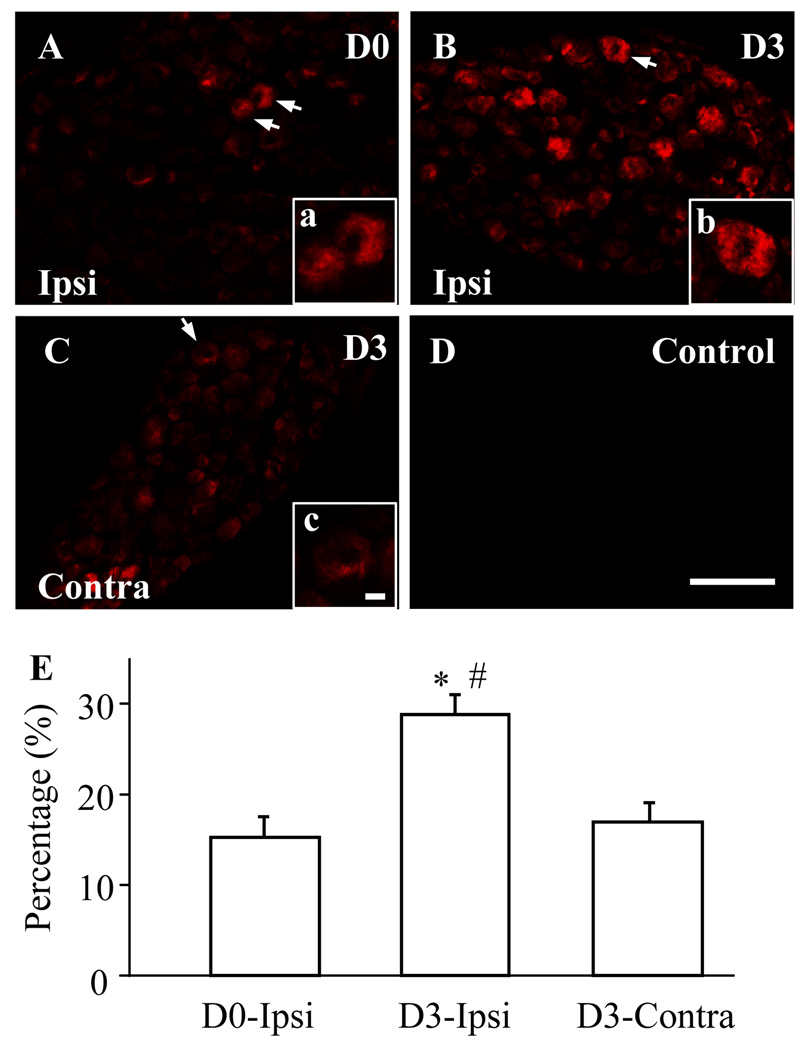
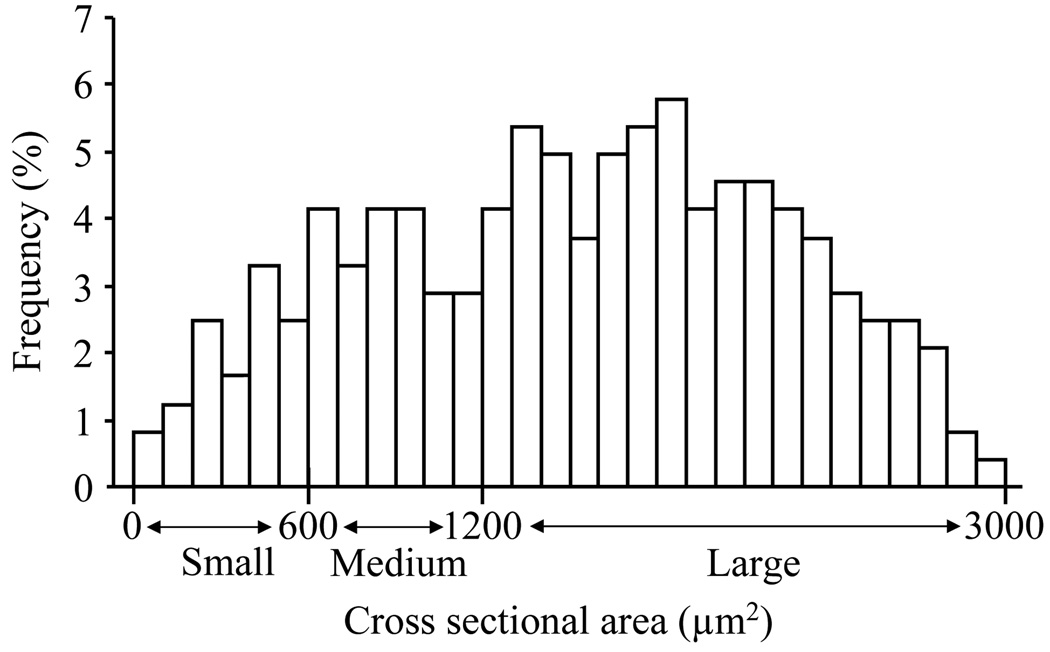
We first examined Nav1.1 protein expression in the DRG at different time points after SNL. We found that the level of Nav1.1 in the ipsilateral L5 DRG was increased by 1.88 fold (± 0.09) on day 3 (n = 9, P < 0.05) and 1.41 fold (± 0.21) on day 7 (n = 9, P < 0.05) after SNL compared to that in naïve rats (n = 9; Fig. 1). No significant change in Nav1.1 expression was observed in the contralateral L5 DRG (Fig. 1). As expected, sham surgery did not alter the basal amount of Nav1.1 in either ipsilateral or contralateral DRGs (data not shown). Because the greatest increase in DRG Nav1.1 expression was observed on day 3 after SNL, the number of Nav1.1-positive DRG cells was examined at this time point. Approximately 15.25 ± 2.32% of DRG neurons were Nav1.1-positive in naïve rats (Fig. 2). Consistent with previous reports (Fukuoka et al., 2008; Ho and O'Leary, 2011), Nav1.1 protein was expressed predominantly in large diameter sensory neurons and to a lesser extent in medium and small diameter neurons (Figs. 2 and 3). On day 3 post-SNL, the percentage of Nav1.1-positive cells in the L5 DRGs was significantly increased on the ipsilateral side (28.95 ± 2.20%, P < 0.05), but not on the contralateral side (17.05 ± 2.13%, P > 0.05), compared to that in naïve rats (Fig. 2). No immuno-signal was detected in the DRG sections when the primary specific antibody was omitted (Fig. 2D).
Fig. 2.
Spinal nerve ligation (SNL) increased the number of Nav1.1-positive neurons in the L5 dorsal root ganglia (DRG). A–C: DRG sections were immunostained with anti-Nav1.1. Representative images of naive DRG (A), ipsilateral (Ipsi) L5 DRG (B), and contralateral (Contra) L5 DRG (C) on day 3 post-SNL. Insets a–c are high magnification images of the of Nav1.1-positive cells indicated by the arrows in A–C, respectively. D: A negative control showing absence of signal in the DRG when primary antibody was omitted. Scale bars: 200 µm in A–D and 20 µm in a–c. E: Statistical summary showing the percentage of Nav1.1-positive neurons in naïve (D0) DRG, ipsilateral L5 DRG (B), and contralateral L5 DRG (C) on day 3 (D3) post-SNL. * P < 0.05 vs the corresponding naive group. # P < 0.05 vs the corresponding contralateral side.
Fig. 3.
Histogram showing the distribution of Nav1.1-positive somata in the DRG. Nav1.1 protein was expressed predominantly in large diameter sensory neurons and to a lesser extent in medium and small diameter neurons.
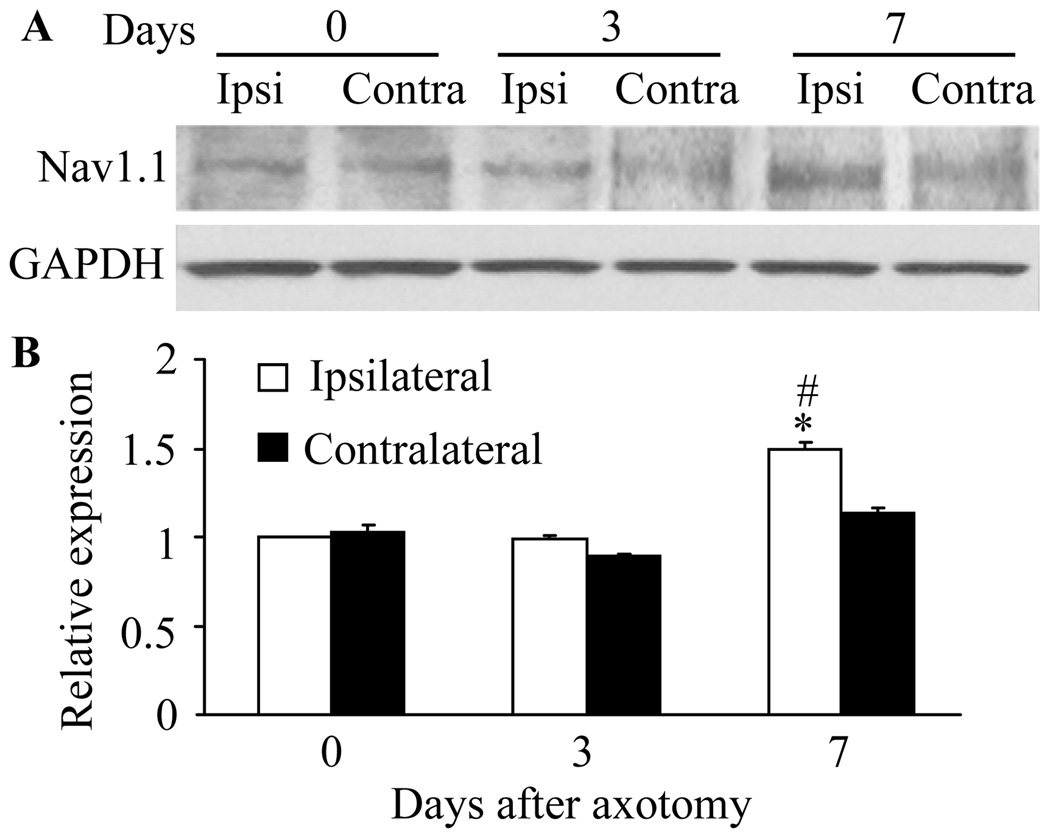
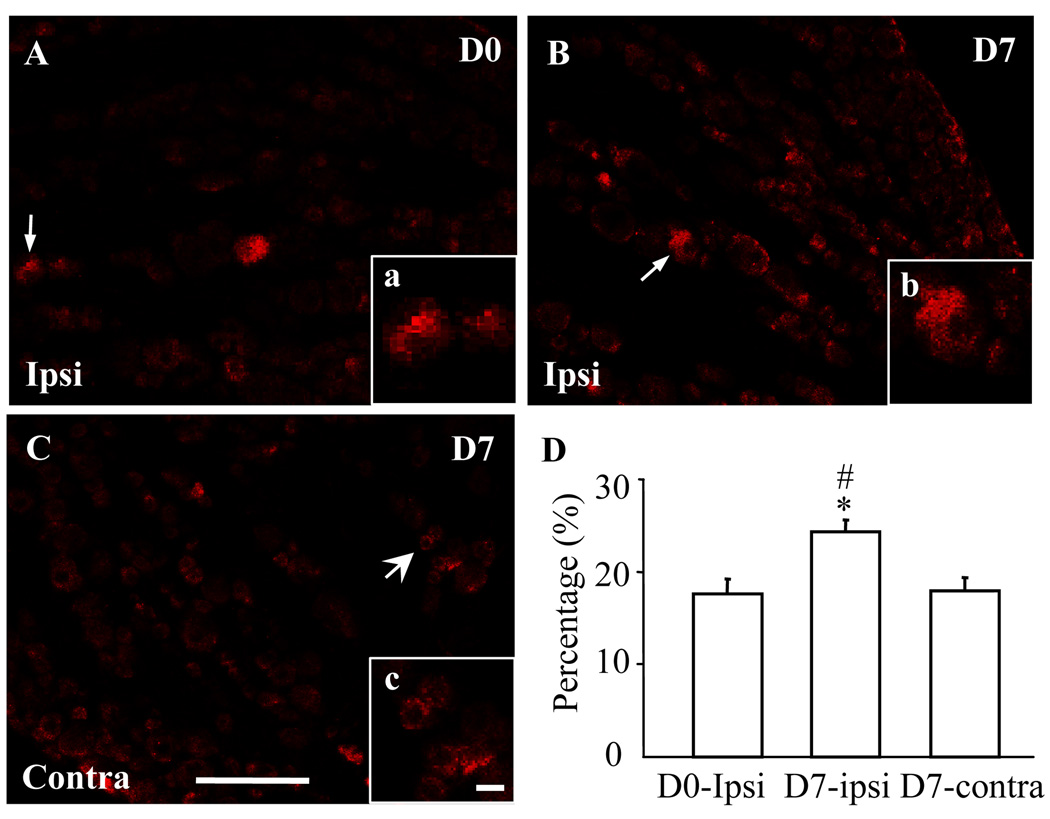
Next we examined the expression of DRG Nav1.1 protein after sciatic nerve axotomy. We found that the expression of Nav1.1 in the injured DRG was unchanged on day 3 (0.98 ± 0.02, n = 9, P > 0.05) but was significantly increased on day 7 (1.49 ± 0.04, n = 9, P < 0.05) post-axotomy, compared to that in naïve rats (n = 9; Fig. 4). No significant changes were observed in the level of Nav1.1 in the contralateral DRG on day 3 or 7 after axotomy (Fig. 4). As expected, sham surgery did not change the basal level of Nav1.1 protein in either ipsilateral or contralateral DRG (data not shown). Immunohistochemistry showed that the percentage of Nav1.1-positive cells in the ipsilateral DRG was dramatically increased on day 7 post-axotomy (24.29 ± 1.31%, P < 0.05, Fig. 5) compared to that in naïve rats (17.58 ± 1.61%). In the contralateral DRG, the percentage of Nav1.1-positive cells on day 7 post-axotomy was 17.87 ± 1.54% (P > 0.05, Fig. 5).
Fig. 4.
Sciatic nerve axotomy increased the level of Nav1.1 protein in the L5 injured dorsal root ganglia (DRG). A: Representative Western blots showing expression of Nav1.1 in the DRG of naïve rats (day 0) and of rats on days 3 and 7 after sciatic nerve axotomy. Ipsi: ipsilateral. Contra: contralateral. B: Statistical summary of the densitometric analysis showing the expression of Nav1.1 relative to that of ipsilateral DRG from naïve rats after normalization to the corresponding glyceraldehyde 3-phosphate dehydrogenase density. * P < 0.05 vs the corresponding naive group. # P < 0.05 vs the corresponding contralateral side.
Fig. 5.
Axotomy increased the number of Nav1.1-positive neurons in the L5 dorsal root ganglia (DRG). Representative images of naive DRG (A), ipsilateral (Ipsi) L5 DRG (B), and contralateral (Contra) L5 DRG (C) on day 7 after sciatic nerve axotomy. Insets a–c are high magnification images of the Nav1.1-positive cells indicated by the arrows in A–C, respectively. Scale bars: 200 µm in A–C and 20 µm in a–c. D: Statistical summary showing the percentage of Nav1.1-positive neurons in naïve (D0) DRG, ipsilateral L5 DRG (B), and contralateral L5 DRG (C) on day 7 (D7) after sciatic nerve axotomy. * P < 0.05 vs the corresponding naive group. # P < 0.05 vs the corresponding contralateral side.
DISCUSSION
In the present study, we used Western blot and immunostaining methods to assess the protein expression of Nav1.1 in the DRGs of rats after peripheral nerve injury (SNL and sciatic nerve axotomy). Our results provide direct evidence that nerve injury induces a significant temporal increase in Nav1.1 protein expression in the injured DRG. This increase implies that DRG Nav1.1 might participate in the induction of neuropathic pain.
The timing of the increase in Nav1.1 expression was different in the SNL and axotomy models. In the SNL model, the increase of Nav1.1 protein in the injured DRG correlates well with the mechanical allodynia observed on days 3 and 7 post-SNL, suggesting that an early increase of Nav1.1 protein may be involved in the induction of nerve injury-induced pain hypersensitivity. Interestingly, DRG Nav1.1 was significantly increased in the sciatic axotomy model on day 7, but not on day 3. The reason of the discrepancy between the two models is unclear, but it might be related to the distinct sites of transection. Sciatic nerve axotomy may take longer to influence protein expression in the DRG compared to spinal nerve injury. For example, Nav1.1 expression might be regulated by translational and other factors that are produced as a result of nerve degradation and regeneration at the injured site (Qiu et al., 2005; Willis et al., 2005; Uchida et al., 2010). Such factors might require more time for retrograde transport to the DRG after axotomy. It should be noted that other potential possibilities cannot be ruled out from the current study as each model, regardless of behavioral output, causes differences in expression in DRG sodium channels (Wang et al., 2011).
Using a real-time reverse transcriptase-PCR approach, previous studies have shown the level of Nav1.1 mRNA to be decreased, not increased, in the injured DRG after SNL (Kim et al., 2001; Berta et al., 2008). We expected that changes in the protein level would parallel those at the mRNA level. However, two different experimental approaches (Western blot and immunohistochemistry) revealed a significant increase in Nav1.1 protein in the injured DRG after both peripheral nerve injury models. It is unknown why the changes in mRNA and protein levels are inconsistent. A previous in situ hybridization histochemistry study showed that more than 25% of the DRG neurons in naïve rats were positive for Nav1.1 mRNA (Fukuoka et al., 2008), whereas we found that only 15% of DRG neurons in naïve rats were positive for Nav1.1 protein. The results indicate that the Nav1.1 mRNA in some DRG cells might not be translated into protein (i.e., it is silent) and/or it might have a low efficiency of protein translation under normal conditions. The level of Nav1.1 protein in these cells could not be detected by the antibody used in our immunohistochemical study. It is possible that peripheral nerve injury might activate silent Nav1.1 RNA and/or increase the translational efficiency through unknown post-translational mechanisms while down-regulating Nav1.1 transcription. These potential mechanisms will be investigated in our future studies.
The present study showed that peripheral nerve injury up-regulates Nav1.1 protein expression in the injured DRG at early time periods. This increase in protein might be involved in the induction of neuropathic pain. However, the use of strategies to genetically knock down or knock out Nav1.1 will be required to confirm the role of DRG Nav1.1 in the mechanisms that underlie the development and maintenance of neuropathic pain.
Acknowledgements
The authors thank Claire F. Levine, MS, for her editorial assistance.
Grant sponsors: NIH/NINDS (Grant number: NS 058886), Blaustein Pain Research Fund; Mr. David Koch and the Patrick C. Walsh Prostate Cancer Research Fund; the Brain Science Institute at the Johns Hopkins University.
Abbreviations used
- DRG
dorsal root ganglion
- L
lumbar
- PWT
paw withdrawal threshold
- SNL
spinal nerve ligation
LITERATURE CITED
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berta T, Poirot O, Pertin M, Ji RR, Kellenberger S, Decosterd I. Transcriptional and functional profiles of voltage-gated Na(+) channels in injured and non-injured DRG neurons in the SNI model of neuropathic pain. Mol Cell Neurosci. 2008;37:196–208. doi: 10.1016/j.mcn.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. Sodium channels in normal and pathological pain. Annu Rev Neurosci. 2010;33:325–347. doi: 10.1146/annurev-neuro-060909-153234. [DOI] [PubMed] [Google Scholar]
- Dichgans M, Freilinger T, Eckstein G, Babini E, Lorenz-Depiereux B, Biskup S, Ferrari MD, Herzog J, van den Maagdenberg AM, Pusch M, Strom TM. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet. 2005;366:371–377. doi: 10.1016/S0140-6736(05)66786-4. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Kobayashi K, Yamanaka H, Obata K, Dai Y, Noguchi K. Comparative study of the distribution of the alpha-subunits of voltage-gated sodium channels in normal and axotomized rat dorsal root ganglion neurons. J Comp Neurol. 2008;510:188–206. doi: 10.1002/cne.21786. [DOI] [PubMed] [Google Scholar]
- Guan X, Zhu X, Tao YX. Peripheral nerve injury up-regulates expression of interactor protein for cytohesin exchange factor 1 (IPCEF1) mRNA in rat dorsal root ganglion. Naunyn-Schmied Arch Pharmacol. 2009;380:459–463. doi: 10.1007/s00210-009-0451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C, O'Leary ME. Single-cell analysis of sodium channel expression in dorsal root ganglion neurons. Mol Cell Neurosci. 2011;46:159–166. doi: 10.1016/j.mcn.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Oh Y, Chung JM, Chung K. The changes in expression of three subtypes of TTX sensitive sodium channels in sensory neurons after spinal nerve ligation. Brain Res Mol Brain Res. 2001;95:153–161. doi: 10.1016/s0169-328x(01)00226-1. [DOI] [PubMed] [Google Scholar]
- Mo G, Grant R, O'Donnell D, Ragsdale DS, Cao CQ, Seguela P. Neuropathic Nav1.3-mediated sensitization to P2X activation is regulated by protein kinase C. Mol Pain. 2011;7:14. doi: 10.1186/1744-8069-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Cafferty WB, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci. 2005;25:1645–1653. doi: 10.1523/JNEUROSCI.3269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh OV, Yaster M, Xu JT, Guan Y, Guan X, Dharmarajan AM, Raja SN, Zeitlin PL, Tao YX. Proteome of synaptosome-associated proteins in spinal cord dorsal horn after peripheral nerve injury. Proteomics. 2009;9:1241–1253. doi: 10.1002/pmic.200800636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida H, Ma L, Ueda H. Epigenetic gene silencing underlies C-fiber dysfunctions in neuropathic pain. J Neurosci. 2010;30:4806–4814. doi: 10.1523/JNEUROSCI.5541-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahedi K, Depienne C, Le Fort D, Riant F, Chaine P, Trouillard O, Gaudric A, Morris MA, Leguern E, Tournier-Lasserve E, Bousser MG. Elicited repetitive daily blindness: a new phenotype associated with hemiplegic migraine and SCN1A mutations. Neurology. 2009;72:1178–1183. doi: 10.1212/01.wnl.0000345393.53132.8c. [DOI] [PubMed] [Google Scholar]
- Wang W, Gu J, Li YQ, Tao YX. Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain? Mol Pain. 2011;7:16. doi: 10.1186/1744-8069-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D, Li KW, Zheng JQ, Chang JH, Smit A, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Tao F, Liaw WJ, Bredt DS, Johns RA, Tao YX. Effect of knock down of spinal cord PSD-93/chapsin-110 on persistent pain induced by complete Freund’s adjuvant and peripheral nerve injury. Pain. 2003;106:187–196. doi: 10.1016/j.pain.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]