To the Editor:
Smith-Magenis syndrome (SMS; OMIM 182290) is a genomic disorder characterized by multiple congenital anomalies, developmental delay, intellectual disability, and a distinct behavioral phenotype including aggressiveness, self-injury, and sleep disturbances [Edelman et al., 2007; Greenberg et al., 1996; Smith et al., 1986]. Most patients with SMS (~75%) harbor a recurrent 3.7 megabase (Mb) microdeletion of 17p11.2, mediated by flanking low copy repeats (LCRs) [Bi et al., 2003; Chen et al., 1997; Potocki et al., 2003; Shaw et al., 2002]. . The retinoic acid-induced gene 1 (RAI1) is one of approximately 25 genes within the SMS critical region [Bi et al., 2002; Vlangos et al., 2003], and heterozygous RAI1 mutations have been reported in individuals who resemble SMS patients clinically, but who lack a 17p11.2 deletion; thus, haploinsufficiency of RAI1 is considered the cause of most of the manifestations of SMS [Bi et al., 2004, 2006; Girirajan et al., 2005, 2006; Slager et al., 2003]. Nonetheless, the prevalence of some phenotypes, for example cardiovascular malformations and hearing loss, differ between SMS deletion patients and those with RAI1 point mutations [Edelman et al., 2007; Girirajan et al., 2006], suggesting that other loci within 17p11.2 and/or genomic rearrangement itself [Ricard et al., 2010] may contribute to or modify the SMS phenotype.
Subjective sleep disturbances are well documented in SMS [Smith et al., 1998]. Additionally, objective sleep disturbances, including multiple awakenings, decreased or increased percentage of REM sleep, and decreased total sleep time have been detected by polysomnography [Greenberg et al., 1996; Potocki et al., 2000] and actigraphy [De Leersnyder et al., 2001a; Gropman et al., 2006]. Inverted circadian rhythmicity of melatonin in SMS deletion patients has also been reported [De Leersnyder et al., 2001a; Potocki et al., 2000].
Persons with RAI1 mutations also have a high rate of subjective sleep disturbance (14 of 14 subjects described in [Bi et al., 2004, 2006; Girirajan et al., 2005, 2006; Slager et al., 2003]), statistically equivalent to that of individuals with the common (3.7 Mb) SMS deletion (39/39 in [Potocki et al., 2003]). It is unknown if RAI1 mutations lead to an alteration of melatonin rhythmicity. Given melatonin’s role in sleep-wake patterning [Brzezinski 1997], we hypothesized that haploinsufficiency of RAI1 alone may lead to inversion of melatonin rhythmicity, potentially explaining disordered sleep in individuals with this genotype. In this report we document an inverted circadian rhythm of melatonin in a child and an adult who each harbor a RAI1 mutation.
Individuals with clinical suspicion for SMS were enrolled in a multidisciplinary clinical study, approved by the Institutional Review Board of Baylor College of Medicine, at Texas Children’s Hospital in Houston, USA; informed consent was provided in each case. This comprehensive clinical protocol included physical examination, polysomnography, and urine collection to measure 6-sulfatoxymelatonin (6-hydroxymelatonin sulfate, aMT6s), the major excreted metabolite of melatonin [Reiter, 1991; Potocki et al., 2000]. Molecular characterization and some phenotypic details of these subjects have been described previously [Bi et al., 2004, 2006; Chen et al., 1997; Liburd et al., 2001; Potocki et al., 2000, 2003; Slager et al., 2003]. Briefly, individuals with clinical suspicion for SMS were screened by FISH for deletions on chromosome 17p11.2. Subjects who did not have a del(17)(p11.2p11.2) by FISH underwent sequencing of the RAI1 gene, which identified a heterozygous nonsense mutation in subject 1106, an 11-year-old girl [Bi et al., 2004], and a heterozygous frameshift mutation in subject 526 (listed as SMS156 in Slager et al., [2003]), a 27-year-old woman. Both of these subjects experienced subjective sleep disturbances by parental report. Subject 1106 took melatonin (6 mg at bedtime), which was discontinued 3 weeks prior to the study. Subject 526 took no medications.
Subjects were evaluated in the sleep laboratory at Texas Children’s Hospital [Potocki et al., 2000]. Briefly, the subjects underwent continuous 21-channel polysomnographic monitoring as well as in-person and video behavioral monitoring for one night. Sleep staging was determined using standard criteria. A multiple sleep latency test (MSLT) was performed the following day. The subjects’ spontaneously voided urine was collected at several time points and analyzed for aMT6s concentration [Potocki et al., 2000]. Urine from a healthy girl and a healthy man, analyzed similarly, served as controls [Reiter et al., 1996].
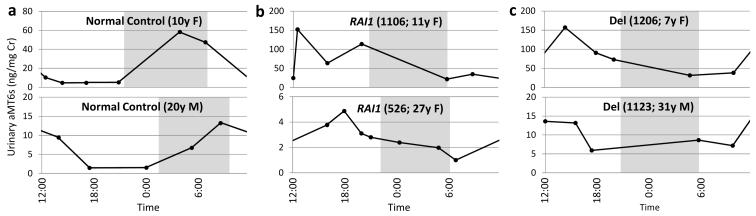
Both subjects with RAI1 mutations demonstrated sleep disturbances by polysomnography similar to those of subjects with the SMS common deletion (Table I). MSLT findings were normal (data not shown). The rhythm of urinary aMT6s concentration, a surrogate for serum melatonin concentration [Reiter, 1991], was found to be altered in both subjects with RAI1 mutations (Fig. 1). A normal circadian rhythm of urinary aMT6s concentration peaks in the early morning (Fig. 1a), resulting from a nocturnal maximum of melatonin production. In contrast, the urinary aMT6s concentration in both child (1106) and adult (526) with RAI1 point mutations (Fig. 1b) displayed an inverted pattern with a daytime maximum, similar to age-matched subjects with the SMS common deletion (1206 and 1123, respectively [Potocki et al., 2000]) shown for comparison (Fig. 1c). As subjects 1106 and 1206 and the younger control individual are children, their overall levels of aMT6s are expectedly higher than those of the adult control individual or of subjects 526 and 1123 [Waldhauser et al., 1984].
Table I.
Sleep abnormalities in four subjects.
| Patient No. |
Age | Gender | Molecular Diagnosis |
Polysomnographic Abnormalities | Melatonin Rhythm |
References |
|---|---|---|---|---|---|---|
| 1106 | 11y | F |
RAI1 mutation C2878T (nonsense) |
Multiple nocturnal awakenings (4, including one of 1h 45m), mildly decreased total sleep time (6h 57m), increased percentage REM sleep (32%) |
Inverted | [Bi et al., 2004, 2006] |
| 526 | 27y | F |
RAI1 mutation 5265delC (frameshift) |
Multiple complete or partial arousals (15), one brief central apneic event (10s) associated with oxygen desaturation (to 87%) |
Inverted | [Bi et al., 2004, 2006; Edelman et al., 2007; Girirajan et al., 2006; Slager et al., 2003] a |
| 1206 | 7y | F |
Common SMS
deletion |
Multiple nocturnal awakenings (18), low percentage REM sleep (16%) |
Inverted | [Potocki et al., 2000, 2003] |
| 1123 | 31y | M |
Common SMS
deletion |
Multiple nocturnal awakenings (7), low total sleep time (4h 32m), low percentage REM sleep (1%) |
Inverted | [Chen et al., 1997; Liburd et al., 2001; Potocki et al., 2000, 2003] |
This patient reported in [Slager et al., 2003] as SMS156 (4929delC).
MSLT, multiple sleep latency test; REM, rapid eye movement.
Figure 1. Melatonin rhythmicity is altered in RAI1 mutation patients.
Levels of urinary 6-sulfatoxymelatonin (aMT6s), a surrogate for serum melatonin concentration, were determined over one day and normalized to urinary creatinine (Cr). a. In healthy individuals, the highest concentration of aMT6s is found in the first morning sample, reflecting the normal rise of serum melatonin during the night (adapted from [Reiter et al., 1996]). b-c. This rhythmicity is ‘inverted’ in RAI1 mutation patients (b), similar to individuals with the SMS common deletion (c; adapted from [Potocki et al., 2000]). Both children (top) and adult patients (bottom) exhibit this aberrant rhythmicity. The overall magnitude of melatonin concentrations is expectedly higher in children than in adult subjects [Waldhauser et al., 1984]. Shaded areas indicate the period of darkness. RAI1, RAI1 mutation; Del, common SMS deletion.
Subjective sleep disturbance is common in SMS, corroborated by objective abnormalities on polysomnography [Greenberg et al., 1991, 1996]. SMS deletion patients have been reported to have an inverted circadian rhythm of melatonin [De Leersnyder et al., 2001a; Potocki et al., 2000]. Our results indicate that SMS patients with mutations in RAI1, a gene mapping within the SMS critical region, have similarly altered melatonin rhythmicity.
It is unknown whether RAI1 is the only gene within the SMS critical region for which reduced dosage leads to altered melatonin rhythmicity. For example, RASD1, also located within the SMS critical region, is a modulator of the responsiveness of the core (suprachiasmatic nucleus) circadian clock to photic and nonphotic inputs [Cheng et al., 2004]. It is also unknown 1) whether alteration of melatonin rhythmicity is solely responsible for the sleep disturbances in SMS, 2) whether this alteration accompanies or causes similar disturbance of the core circadian clock, peripheral clocks, or other clock outputs, and 3) most basically, whether it constitutes a true inversion of rhythmicity or simply a phase advance or delay of approximately half of a day.
Properly-timed exogenous administration of melatonin can shift human circadian rhythms [Lewy et al., 1992] and promote and quantitatively improve sleep in adults with sleep disorders and in children with neurodevelopmental difficulties [Brzezinski 1997; Jan et al., 2000; Zhdanova and Wurtman 1997]. Thus, exogenous melatonin has been given nocturnally to SMS patients to induce and improve sleep, in some cases in combination with diurnal β1-adrenergic antagonist administration to inhibit daytime melatonin production and resultant daytime sleepiness [Carpizo et al., 2006; De Leersnyder et al., 2001b, 2003, 2006]. The success of this regimen in a small number of patients is promising, and our results indicate that, in addition to SMS deletion patients, it is rational that RAI1 mutation patients may also benefit from such treatments.
ACKNOWLEDGMENTS
We are indebted to the subjects who participated in this study. We thank Marjorie Withers, Doreen Osterholm, and Anita Thompson, REEGT, for technical assistance. We thank Dianne Dang for assistance in composing the manuscript and Dr. Wenli Gu for helpful revisions. P.M.B. is supported by a National Eye Institute training grant (T32EY007102) from the United States National Institutes of Health (NIH). This work was supported in part by a National Institute of Neurological Disorders and Stroke grant (R01NS058529) from the NIH (J.R.L.), by a National Institute of Child Health and Development grant (K08HD01149) from the NIH (L.P.), by the Baylor College of Medicine Intellectual and Developmental Disabilities Research Center (HD2406402), and by the Texas Children’s Hospital General Clinical Research Center (M01RR00188). J.R.L. is a paid consultant for Athena Diagnostics and Ion Torrent Systems and is a co-inventor on multiple United States and European patents related to molecular diagnostics. The Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from the chromosomal microarray analysis offered in the Medical Genetics Laboratory.
REFERENCES
- Bi W, Park S-S, Shaw CJ, Withers MA, Patel PI, Lupski JR. Reciprocal crossovers and a positional preference for strand exchange in recombination events resulting in deletion or duplication of chromosome 17p11.2. Am J Hum Genet. 2003;73:1302–1315. doi: 10.1086/379979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi W, Saifi GM, Girirajan S, Shi X, Szomju B, Firth H, Magenis RE, Potocki L, Elsea SH, Lupski JR. RAI1 point mutations, CAG repeat variation, and SNP analysis in non-deletion Smith-Magenis syndrome. Am J Med Genet A. 2006;140A:2454–2463. doi: 10.1002/ajmg.a.31510. [DOI] [PubMed] [Google Scholar]
- Bi W, Saifi GM, Shaw CJ, Walz K, Fonseca P, Wilson M, Potocki L, Lupski JR. Mutations of RAI1, a PHD-containing protein, in nondeletion patients with Smith-Magenis syndrome. Hum Genet. 2004;115:515–524. doi: 10.1007/s00439-004-1187-6. [DOI] [PubMed] [Google Scholar]
- Bi W, Yan J, Stankiewicz P, Park SS, Walz K, Boerkoel CF, Potocki L, Shaffer LG, Devriendt K, Nowaczyk MJ, Inoue K, Lupski JR. Genes in a refined Smith-Magenis syndrome critical deletion interval on chromosome 17p11.2 and the syntenic region of the mouse. Genome Res. 2002;12:713–728. doi: 10.1101/gr.73702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- Carpizo R, Martínez Á , Mediavilla D, González M, Abad A, Sánchez-Barceló EJ. Smith-Magenis syndrome: a case report of improved sleep after treatment with β1-adrenergic antagonists and melatonin. J Pediatr. 2006;149:409–411. doi: 10.1016/j.jpeds.2006.04.055. [DOI] [PubMed] [Google Scholar]
- Chen K-S, Manian P, Koeuth T, Potocki L, Zhao Q, Chinault AC, Lee CC, Lupski JR. Homologous recombination of a flanking repeat gene cluster is a mechanism for a common contiguous gene deletion syndrome. Nat Genet. 1997;17:154–163. doi: 10.1038/ng1097-154. [DOI] [PubMed] [Google Scholar]
- Cheng H-YM, Obrietan K, Cain SW, Lee BY, Agostino PV, Joza NA, Harrington ME, Ralph MR, Penninger JM. Dexras1 potentiates photic and suppresses nonphotic responses of the circadian clock. Neuron. 2004;43:715–728. doi: 10.1016/j.neuron.2004.08.021. [DOI] [PubMed] [Google Scholar]
- De Leersnyder H. Inverted rhythm of melatonin secretion in Smith-Magenis syndrome: from symptoms to treatment. Trends Endocrinol Metab. 2006;17:291–298. doi: 10.1016/j.tem.2006.07.007. [DOI] [PubMed] [Google Scholar]
- De Leersnyder H, Bresson JL, de Blois M-C, Souberbielle J-C, Mogenet A, Delhotal-Landes B, Salefranque F, Munnich A. β1-adrenergic antagonists and melatonin reset the clock and restore sleep in a circadian disorder, Smith-Magenis syndrome. J Med Genet. 2003;40:74–78. doi: 10.1136/jmg.40.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leersnyder H, Claustrat B, Munnich A, Verloes A. Circadian rhythm disorder in a rare disease: Smith-Magenis syndrome. Mol Cell Endocrinol. 2006;252:88–91. doi: 10.1016/j.mce.2006.03.043. [DOI] [PubMed] [Google Scholar]
- De Leersnyder H, de Blois M-C, Claustrat B, Romana S, Albrecht U, von Kleist-Retzow J-C, Delobel B, Viot G, Lyonnet S, Vekemans M, Munnich A. Inversion of the circadian rhythm of melatonin in the Smith-Magenis syndrome. J Pediatr. 2001a;139:111–116. doi: 10.1067/mpd.2001.115018. [DOI] [PubMed] [Google Scholar]
- De Leersnyder H, de Blois M-C, Vekemans M, Sidi D, Villain E, Kindermans C, Munnich A. β1-adrenergic antagonists improve sleep and behavioural disturbances in a circadian disorder, Smith-Magenis syndrome. J Med Genet. 2001b;38:586–590. doi: 10.1136/jmg.38.9.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman EA, Girirajan S, Finucane B, Patel PI, Lupski JR, Smith ACM, Elsea SH. Gender, genotype, and phenotype differences in Smith-Magenis syndrome: a meta-analysis of 105 cases. Clin Genet. 2007;71:540–550. doi: 10.1111/j.1399-0004.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- Girirajan S, Elsas LJ, 2nd, Devriendt K, Elsea SH. RAI1 variations in Smith-Magenis syndrome patients without 17p11.2 deletions. J Med Genet. 2005;42:820–828. doi: 10.1136/jmg.2005.031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girirajan S, Vlangos CN, Szomju BB, Edelman E, Trevors CD, Dupuis L, Nezarati M, Bunyan DJ, Elsea SH. Genotype-phenotype correlation in Smith-Magenis syndrome: evidence that multiple genes in 17p11.2 contribute to the clinical spectrum. Genet Med. 2006;8:417–427. doi: 10.1097/01.gim.0000228215.32110.89. [DOI] [PubMed] [Google Scholar]
- Greenberg F, Guzzetta V, de Oca-Luna R Montes, Magenis RE, Smith ACM, Richter SF, Kondo I, Dobyns WB, Patel PI, Lupski JR. Molecular analysis of the Smith-Magenis syndrome: a possible contiguous-gene syndrome associated with del(17)(p11.2) Am J Hum Genet. 1991;49:1207–1218. [PMC free article] [PubMed] [Google Scholar]
- Greenberg F, Lewis RA, Potocki L, Glaze D, Parke J, Killian J, Murphy MA, Williamson D, Brown F, Dutton R, McCluggage C, Friedman E, Sulek M, Lupski JR. Multidisciplinary clinical study of Smith-Magenis syndrome (deletion 17p11.2) Am J Med Genet. 1996;62:247–254. doi: 10.1002/(SICI)1096-8628(19960329)62:3<247::AID-AJMG9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Gropman AL, Duncan WC, Smith ACM. Neurologic and developmental features of the Smith-Magenis syndrome (del 17p11.2) Pediatr Neurol. 2006;34:337–350. doi: 10.1016/j.pediatrneurol.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Jan JE, Hamilton D, Seward N, Fast DK, Freeman RD, Laudon M. Clinical trials of controlled-release melatonin in children with sleep-wake cycle disorders. J Pineal Res. 2000;29:34–39. doi: 10.1034/j.1600-079x.2000.290105.x. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Ahmed S, Jackson JM Latham, Sack RL. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9:380–392. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- Liburd N, Ghosh M, Riazuddin S, Naz S, Khan S, Ahmed Z, Riazuddin S, Liang Y, Menon PSN, Smith T, Smith ACM, Chen K-S, Lupski JR, Wilcox ER, Potocki L, Friedman TB. Novel mutations of MYO15A associated with profound deafness in consanguineous families and moderately severe hearing loss in a patient with Smith-Magenis syndrome. Hum Genet. 2001;109:535–541. doi: 10.1007/s004390100604. [DOI] [PubMed] [Google Scholar]
- Potocki L, Glaze D, Tan D-X, Park S-S, Kashork CD, Shaffer LG, Reiter RJ, Lupski JR. Circadian rhythm abnormalities of melatonin in Smith-Magenis syndrome. J Med Genet. 2000;37:428–433. doi: 10.1136/jmg.37.6.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocki L, Shaw CJ, Stankiewicz P, Lupski JR. Variability in clinical phenotype despite common chromosomal deletion in Smith-Magenis syndrome [del(17)(p11.2p11.2)] Genet Med. 2003;5:430–434. doi: 10.1097/01.gim.0000095625.14160.ab. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991;12:151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Barlow-Walden L, Poeggeler B, Heiden SM, Clayton RJ. Twenty-four hour urinary excretion of 6-hydroxymelatonin sulfate in Down syndrome subjects. J Pineal Res. 1996;20:45–50. doi: 10.1111/j.1600-079x.1996.tb00238.x. [DOI] [PubMed] [Google Scholar]
- Ricard G, Molina J, Chrast J, Gu W, Gheldof N, Pradervand S, Schütz F, Young JI, Lupski JR, Reymond A, Walz K. Phenotypic consequences of copy number variation: insights from Smith-Magenis and Potocki-Lupski syndrome mouse models. PLoS Biol. 2010;8:e1000543. doi: 10.1371/journal.pbio.1000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw CJ, Bi W, Lupski JR. Genetic proof of unequal meiotic crossovers in reciprocal deletion and duplication of 17p11.2. Am J Hum Genet. 2002;71:1072–1081. doi: 10.1086/344346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slager RE, Newton TL, Vlangos CN, Finucane B, Elsea SH. Mutations in RAI1 associated with Smith-Magenis syndrome. Nat Genet. 2003;33:466–468. doi: 10.1038/ng1126. [DOI] [PubMed] [Google Scholar]
- Smith ACM, Dykens E, Greenberg F. Sleep disturbance in Smith-Magenis syndrome (del 17 p11.2) Am J Med Genet (Neuropsychiatr Genet) 1998;81:186–191. [PubMed] [Google Scholar]
- Smith ACM, McGavran L, Robinson J, Waldstein G, Macfarlane J, Zonona J, Reiss J, Lahr M, Allen L, Magenis E. Interstitial deletion of (17)(p11.2p11.2) in nine patients. Am J Med Genet. 1986;24:393–414. doi: 10.1002/ajmg.1320240303. [DOI] [PubMed] [Google Scholar]
- Vlangos CN, Yim DKC, Elsea SH. Refinement of the Smith-Magenis syndrome critical region to ~950kb and assessment of 17p11.2 deletions. Are all deletions created equally? Mol Genet Metab. 2003;79:134–141. doi: 10.1016/s1096-7192(03)00048-9. [DOI] [PubMed] [Google Scholar]
- Waldhauser F, Weiszenbacher G, Frisch H, Zeitlhuber U, Waldhauser M, Wurtman RJ. Fall in nocturnal serum melatonin during prepuberty and pubescence. Lancet. 1984;1:362–365. doi: 10.1016/s0140-6736(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Wurtman RJ. Efficacy of melatonin as a sleep-promoting agent. J Biol Rhythms. 1997;12:644–650. doi: 10.1177/074873049701200620. [DOI] [PubMed] [Google Scholar]