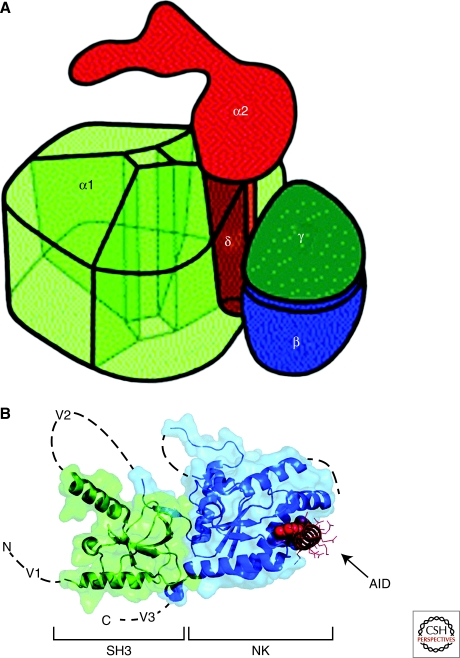
Figure 3.
Three-dimensional architecture of Ca2+ channels. (A) Illustration of the skeletal muscle CaV1.1 channel based on cryo-electronmicroscopy. This drawing assumes pseudo-fourfold symmetry of the α1 subunit. The view shows the extracellular side with the α2 subunit. The α1, γ, and δ subunits are embedded into the lipid membrane (not shown), which separates the extracellular α2 subunit from the cytosol. α2 is anchored via the disulfide-linked δ subunit within the α1 subunit. The proposed model allows for a tight interaction between α1 and δ as well as α1 and γ. (B) Structure of the CaVβ subunit with the α interaction domain (AID). Coordinates are for the CaVβ2a–CaV1.2 AID complex with SH3 (green) and NK (blue) domains are indicated. V1, V2, and V3 show the locations of the three variable domains that are absent from the structure. The AID (red) binds to a deep groove in the NK domain. AID residues tyrosine, tryptophan, and isoleucine are shown as CPK. The remaining residues are shown as lines.