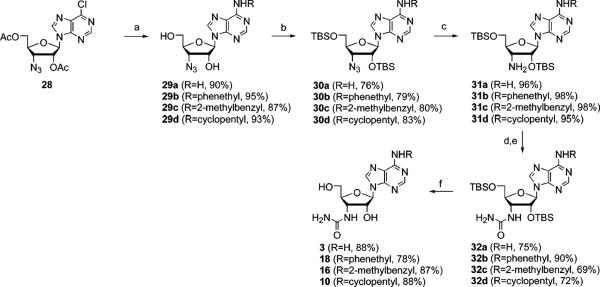
Scheme 2.
Procedure for the Synthesis of 3′-Urea Derivatives of Adenosinea
aReagents and conditions: (a) NH4OH, 1,4-dioxane, room temperature, 24 h, or 2-phenylethylamine, Et3N, EtOH, 50 °C, 18 h, 2-methylbenzylamine, Et3N, EtOH, 50 °C, 18 h, or cyclopentylamine, Et3N, EtOH, 50 °C, 18 h, then NaOMe, MeOH, room temperature, 2 h; (b) TBSCl, imidazole, DMF, room temperature, 24 h; (c) Ph3P, NH4OH/H2O, THF, room temperature, 18 h; (d) chloroacetyl isocyanate, DMF, 0 °C, 3 h; (e) NaOMe, MeOH, room temperature, 18 h; (f) TBAF, THF, room temperature, 4 h.