Abstract
G protein-coupled receptors, the largest cell surface receptor family, have emerged as critical players in cell death and survival. High gene expression level of the Gq-coupled P2Y1 nucleotide receptor in PC-3 prostate cancer cells was demonstrated using real-time quantitative PCR and confirmed by Western blotting and confocal laser scanning microscopy. A selective P2Y1 receptor agonist, the ADP analogue MRS2365, concentration-dependently induced intracellular calcium mobilization (EC50 5.28 nM), which was diminished by P2Y1 receptor-selective antagonist MRS2500. P2Y1 receptor activation by MRS2365 induced apoptosis in assays of Caspase-3, LDH release, and Annexin-V staining. The pro-apoptotic effect of MRS2365 was blocked by MRS2500, P2Y1 siRNA, and an inhibitor of the MAP kinase pathway PD98059. MRS2365 significantly inhibited the proliferation of PC-3 cells, examined using a MTT assay. Thus, activation of the P2Y1 receptor induced cell death and inhibited growth of human prostatic carcinoma PC-3 cells. Activation of the P2Y1 receptor should be a novel and promising therapeutic strategy for prostate cancer.
Keywords: Prostate cancer, P2Y1 receptor, apoptosis, nucleotide, GPCR
1. Introduction
G protein-coupled receptors (GPCRs), the largest family of cell surface receptors, are known to modulate most physiological functions, but their roles in cancer progression and treatment are often not fully appreciated [1,2]. However, an increasing number of GPCRs have recently been shown to be crucial players in tumor growth and metastasis and thus are emerging targets for cancer [1,2]. GPCRs that respond to extracellular nucleotides consist of an eight-member family comprising P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11–14 receptors [3]. The potential roles of nucleotides in various cancers have recently been reviewed [4].
P2Y1, P2Y12 and P2Y13 receptors are activated by the same endogenous agonist, ADP, and are involved in many important physiological functions [3,5,6]. Isolated pieces of evidence also showed that the P2Y1 receptor regulates cell death and growth. For example, in 1321N1 astrocytoma cells transfected with the human P2Y1 receptor, a non-selective P2Y1 agonist 2-MeSADP was able to induce apoptosis and inhibit proliferation by activating this receptor [7,8]. In a later study using A375 melanoma cells, ATP diminished cell proliferation, an effect blocked by a P2Y1 receptor antagonist MRS2179, suggesting an anti-proliferative effect of the P2Y1 receptor [9,10]. In addition to the P2Y1 receptor, other P2Y receptor subtypes, such as the Gq-coupled P2Y2, P2Y6, and P2Y11 receptors and the Gi-coupled P2Y12 and P2Y13 receptors, have also been reported to be relevant to cell death or growth [8,11–15].
The role of P2Y1 receptor signaling in the prostate has not previously been well explored, although it was demonstrated that the P2Y1 receptor was most abundantly expressed in the human prostate [16]. In the present study, we used PC-3 prostate cancer cells as a model to investigate if the P2Y1 receptor is involved in cell death and growth. We found that the expression level of the P2Y1 receptor was highest among the three subtypes of P2Y receptors that respond to ADP. We subsequently probed P2Y1 receptor signaling using a selective P2Y1 receptor agonist, the ADP analogue MRS2365 [17], and found that activation of the P2Y1 receptor induced apoptosis and inhibited cell proliferation, implying a potentially novel target for prostate cancer.
2. Materials and methods
2.1. Materials
The human PC-3 prostate cancer cell line was purchased from American Type Culture Collection (Manassas, VA). RPMI-1640 Medium and fetal bovine serum (FBS) were purchased from Life Technologies (Rockville, MD). TNF-α, ADP, 2-MeSADP, ATP, and cycloheximide, were purchased from Sigma (St. Louis, MO). P2Y1 receptor antagonist MRS2500 ((1R,2S,4S,5S)-4-[2-iodo-6-(methylamino)-9H-purin-9-yl]-2-(phosphonooxy)bicyclo[3.1.0]hexane-1-methanol dihydrogen phosphate ester tetraammonium salt) [18] and agonist MRS2365 ([[(1R,2R,3S,4R,5S)-4-[6-amino-2-(methylthio)-9H-purin-9-yl]-2,3-dihydroxybicyclo[3.1.0]hex-1-yl]methyl] diphosphoric acid mono ester trisodium salt) were purchased from Tocris Biosciences (Ellisville, MO). Predesigned small interfering RNA (siRNA) for P2Y1 receptors, negative control siRNA and SYBR© Green reagents were purchased from Applied Biosystems (Foster City, CA). Annexin V-FITC and Hoechst Stain solution were purchased from Sigma (St. Louis, MO). Horseradish peroxidase (HRP)-linked goat anti-rabbit IgG antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The rabbit polyclonal antibodies for P2Y1 receptor were purchased from Alomone Labs, Ltd. (Jerusalem, Israel). Calcium-4 Mobilization Assay Kit was purchased from Molecular Devices (Sunnyvale, CA). MAP kinase assay kits and Caspase-3 Colorimetric Detection Kit were from Assay Designs (Ann Arbor, MI). Lactate Dehydrogenase (LDH) Assay Kit was purchased from Roche Applied Science (Indianapolis, IN). MTT-based Toxicology Assay Kit was purchased from Sigma-Aldrich (St. Louis, MO). 2-(2-Amino-3-methoxyphenyl)-4H-1-benzopyran-4-one (PD 98059) was purchased from Tocris. All other reagents were from standard sources and are of analytical grade.
2.2. Cell culture
Human prostatic carcinoma PC-3 cells were cultured at 37°C in a humidified incubator with 5% CO2 in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin, and 3 mM/L glutamine. The cells were passaged using trypsinization every 3–5 days.
2.3. Detection of gene expression of P2Y1 receptors in PC-3 cells
The cellular total RNA was isolated from PC-3 cells following the protocol of the RNeasy Mini Kit (Qiagen, Valencia, CA) along with DNase digestion using RNase free DNase (Qiagen). Reverse transcription was completed using Superscript III First Strand Synthesis Supermix kit (Invitrogen, Carlsbad, CA). The cDNA then was amplified by PCR with gene-specific primers for P2Y1 and GAPDH on a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol using SYBR Green PCR MasterMix. Fast amplification parameters were as follows: one cycle of 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec, and 60°C for 1 min. The primers were synthesized by Eurofins MWG Operon (Huntsville, AL) and the sequences as follows: P2Y1: Forward: 5′-CGTGCTGGTGTGGCTCATT-3′; Reverse: 5′-GGACCCCGGTACCTGAGTAGA-3′; P2Y12: Forward: 5′-AGGTCCTCTTCCCACTGCTCTA-3′; Reverse: 5′-CATCGCCAGGCCATTTGT-3′; P2Y13: Forward: 5′-GAGACACTCGGATAGTACAGCTGGTA-3′; Reverse:5′-GCAGGATGCCGGTCAAGA-3′; GAPDH: Forward: 5′-CCACCCATGGCAAATTCC-3′; Reverse: 5′-TGGGATTTCCATTGATGACAAG-3′. Quantitative analysis of data was performed by using the ΔΔCt method [19]. Values were normalized to GAPDH and were expressed as relative expression levels.
2.4. Western blotting
Cells were washed twice with PBS and lysed in 1 ml cell lysis buffer (1x PBS, pH 7.4, 1% Triton X-100, 0.1 mmol/l EDTA, and complete proteinase inhibitor cocktail (Roche)) on ice for 10 min. The cell lysates were centrifuged and supernatants were collected and protein concentration was measured. Each of the 20 μg protein samples was separated by electrophoresis through a 4–12% SDS-PAGE gel and analyzed by Western blotting. Anti-P2Y1 primary antibody (Alomone Labs. Ltd., Israel) was used at 1:200 dilution. Goat anti-rabbit secondary antibodies (Santa Cruz Biotechnology, CA) were used at 1:5000 dilution. The membrane was visualized by exposure to Kodak XAR film.
2.5. Laser confocal microscopy
PC-3 cells and control 1321N1 cells were seeded on cover slips in 6 well plates for two days. Cells were washed once with PBS, and then fixed with cold fix solution (50% methanol/50% acetone) for 20 min at −20°C. After washing with PBS, cells were blocked with 10% FBS 1 h at RT. Anti-P2Y1 antibody (Alomone Labs Ltd, Israel.) was added at 1:200 dilution and incubated with the cells for 1 h. Cells were then washed 3 times with PBS before adding Texas Red goat anti-rabbit IgG (Invitrogen, Carlsbad, CA) and incubated for another 1 h. This was followed by washing three times with PBS, and mounting with ProLong® Gold antifade mounting reagent with DAPI (Invitrogen, Carlsbad, CA). Fluorescence images were obtained with a laser scanning Zeiss LSM-510 Meta Confocal Microscope (Carl Zeiss Inc., Jena, Germany).
2.6. Calcium mobilization assay
Cells were grown overnight in 100 μl of medium in 96-well flat bottom plates at 37°C at 5% CO2 until they reached 80% confluency at a density of 40,000 cells/well. The RPMI-1640 medium in each well was replaced by 30 μl Calcium-4 dye (Molecular Devices Sunnyvale, CA) with no washing of cells and incubated for 1 h at room temperature protected from light. The compound plate was prepared with dilutions of various compounds in Hank’s Balanced Buffer at pH 7.2. The change in calcium was measured by the addition of P2Y1 agonist or antagonist to the dye, and the change in calcium was measured by change in intracellular fluorescence. Samples were run in duplicate with a FLIPRTetra (Molecular Devices, CA) at room temperature. Cell fluorescence (excitation = 485 nm; emission = 525 nm) was monitored following exposure to a compound. Increases in intracellular calcium are reported as the maximum fluorescence value after exposure minus the basal fluorescence value before exposure.
2.7. LDH release assay
PC-3 cells were cultured in 24-well plate at 37°C overnight with complete medium and 100 μl fresh medium with 1% serum was then added to each well. Increasing concentrations of each agonist, diluted with PBS containing calcium and magnesium, and cycloheximide (CHX, 10 μg/ml) were incubated with the cells at 37°C for 24 h. If an antagonist was used, it was incubated with the cells 20 min prior to the addition of the agonist. In all cases, cells that were not treated with CHX, an inhibitor of protein synthesis, served as a control. For the measurement, culture medium was centrifuged and 100 μl supernatant was carefully transferred to corresponding wells of an optically clear 96-well flat bottom microplate in triplicate. LDH activity was measured using a Cytotoxicity Detection kit (Roche Applied Science, Indianapolis, IN) following the manufacturer’s instructions. The absorbance of the samples was measured at 490 nm using a SpectraMax5 Microplate reader (Molecular Devices, Sunnyvale, CA). Cell death was assessed based on release of LDH.
2.8. Cell proliferation assay
PC-3 cells were seeded in 96-well plates at a concentration of 1×104 cells per well in a volume of 100 μl of cell culture medium with 10% serum per well and cultured at 37°C overnight. The P2Y1-receptor agonist MRS2365 (1 μM) was incubated with the cells at 37°C in the CO2 incubator for 24, 48, 72 h. Cell viability was measured using MTT Toxicology Assay Kit (Sigma-Aldrich, St. Louis, MO) following the manufacturer’s protocol. The absorbance was quantified by measuring at 570 nm with a SpectraMax5 Microplate reader (Molecular Devices, Sunnyvale, CA).
2.9. Assay of Caspase-3 activity
PC-3 cells were plated in six-well plates at a density of 2×105 cells per well and cultured to 70% confluence for the experiments. The cells were treated with the P2Y1 receptor agonist MRS2365 in the presence and absence of CHX in the medium for 8 h. CHX was added to the medium 60 min prior to the P2Y1 agonist with a final concentration of 10 μg/ml. Cells were lysed in a RIPA lysis buffer containing protease inhibitors, and protein concentration was quantified. Caspase-3 activity was measured using the Caspase-3 Colorimetric Assay Kit (Enzo Life Sciences, Plymouth Meeting, PA) following the manufacturer’s instructions. Each sample contained 50–200 μg protein, and the absorbance in each well was measured at 405 nm with a microplate reader. Data were plotted and slopes calculated along the linear portion of the curve (at least three separate measurements).
2.10. Assessment of apoptosis by flow cytometry
PC-3 cells (5×105) were treated with the P2Y1 agonist MRS2365 in the presence of CHX for 4 or 8 h. After washing twice with PBS, cells were then resuspended in 100 μl binding buffer with 5 μl annexin V-FITC and 5 μl 7-aminoactinomycin D (7-AAD, labels GC-rich regions of DNA in permeabilized cells) and incubated for 15 min. After an additional 200 μl binding buffer was added, cells were analyzed by Becton-Dickinson FACSCalibur flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ). FITC-labeled annexin V was detected in the FL-1 channel (530 nm), while 7-AAD was detected in the FL-3 channel (650 nm). Data was collected on a log scale and analyzed using CellQuest Pro software.
2.11. P2Y1 receptor siRNA inhibition assay
Predesigned siRNAs (Applied Biosystems, Foster City, CA) against human P2Y1 receptors were used. Their sequences were as follows: sense, GCCCUGAUCUUCUACUACUTT; antisense, AGUAGUAGAAGAUCAGGGCTG. Cells were transfected at about 50–60% confluency by addition of siRNA (1 μM) together with Lipofectamine 2000 transfection reagent as instructed by the manufacturer (Invitrogen, Carlsbad, CA). Cells were split to 6-well plates 24 h after transfection and treated with drugs after an additional 24 h. LDH release was measured after an additional 24 h incubation period.
2.12. Activation of MAP kinases
The procedure used was similar to our previously described [20]. Cells were serum-starved for 4 h before incubation with serum media containing various pharmacological reagents for the appropriate times at 37°C. Reactions were terminated by removing the media and adding 100 μl of RIPA buffer containing proteinase inhibitors. ERK1/2 activity was measured with an ERK1/2 Colorimetric Detection Kit were from Assay Designs (Ann Arbor, MI) according to the manufacture’s instructions.
2.13. Statistical analysis
EC50 values were calculated with Prism 4 (GraphPad, San Diego, CA). Data were analyzed by analysis of variance (ANOVA) (followed by post-hoc analysis) or via Student’s t-test to check the statistical difference among groups with P value less than 0.05 being considered significant. Results were expressed as mean ± S.E.
3. Results
3.1. Detection of the gene expression level of P2Y1 receptors in PC-3 prostate cancer cells
We compared the gene expression levels of three P2Y receptor subtypes, P2Y1, P2Y12 and P2Y13, which respond to ADP, using real-time RT-PCR analysis [19]. Fig. 1 shows that the gene expression level of the P2Y1 receptor message was the highest. The expression levels of message of P2Y12 and P2Y13 receptors were only 7.70 ± 1.62% and 7.43 ± 1.32% of the level of P2Y1 receptor message, respectively. The expression level of the P2Y1 receptor in 1321N1 astrocytoma cells was 8.84 ± 1.64% of that in PC-3 cells (Fig. 1).
Figure 1.

Gene expression levels of three P2Y receptors responding to ADP in comparison to the P2Y1 receptor expression in 1321N1 astrocytoma cells. Total RNA from PC-3 cells or 1321N1 astrocytoma cells was extracted and reverse-transcripted to cDNA and then amplified with gene-specific primers for P2Y1, P2Y12, P2Y13 or GAPDH on a 7900HT Fast Real-Time PCR System. The sequences of primers were listed in the text. Quantitative analysis of data was performed by using the ΔΔCt method [19]. Results are expressed as mean ± SE from three separate experiments. Values were normalized to GAPDH and were expressed as relative expression levels.
3.2. Detection of P2Y1 receptor with Western blotting and laser confocal microscopy
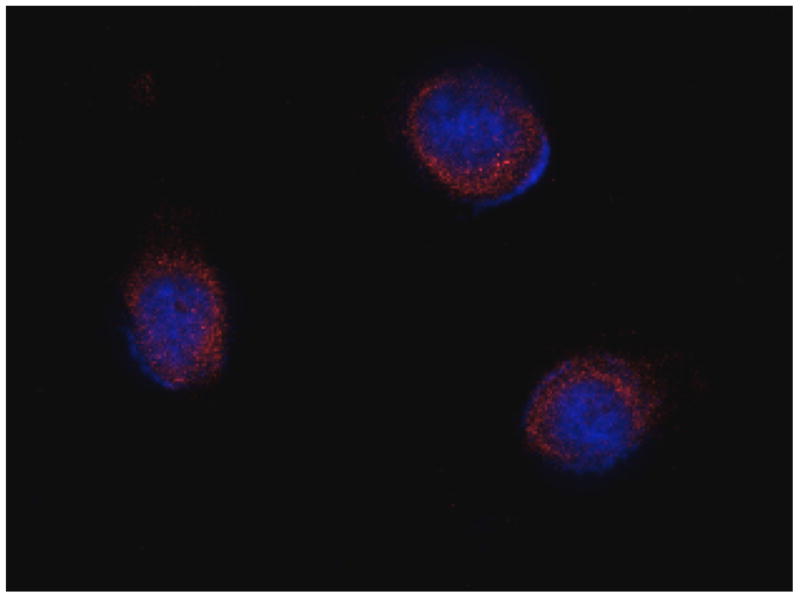
The expression of the P2Y1 receptor was further confirmed with Western blot. Fig. 2A shows that the abundant expression of the P2Y1 receptor (around 90 kDa) was detected in PC-3 prostate cancer cells. 1321N1 astrocytoma cells were used as a control.
Figure 2.
A. Determination of the P2Y1 receptor expression in PC-3 cells using Western blotting. Anti-P2Y1 primary antibody was used at 1:200 dilution. Goat anti-rabbit secondary antibodies were used at 1:5000 dilution. B and C. Localization of P2Y1 receptor expression in control 1321N1 astrocytoma cells and PC-3 cells by confocal laser scanning microscopy. The primary anti-P2Y1 primary antibody was used at 1:200. Texas red secondary antibody was used at 1:400.
The expression of P2Y1 receptors in PC-3 cells was also visualized with laser confocal microscopy. Fig. 2A shows that the majority of the anti- P2Y1 receptor antibody staining locates on the surface of the PC-3 cells in contrast to the control 1321N1 astrocytoma cells (Fig. 2B) which shows a low level of expression of the human P2Y1 receptor.
3.3. Intracellular calcium mobilization assay
The ability of the P2Y1 receptor to mediate intracellular calcium mobilization in PC-3 cells was tested using a FLIPRTetra (Molecular Devices, CA). The potent and selective P2Y1 receptor agonist MRS2365 [17] concentration-dependently induced calcium mobilization corresponding to an EC50 value of 5.28 ± 1.96 nM. A potent and selective P2Y1 receptor antagonist, the bisphosphate derivative MRS2500 (10 nM) [18], rightward-shifted the concentration-response curve of MRS2365 (Fig. 3A). The EC50 value of MRS2365 in the presence of 10 nM MRS2500 was 78.8 ± 16.3 nM.
Figure 3.
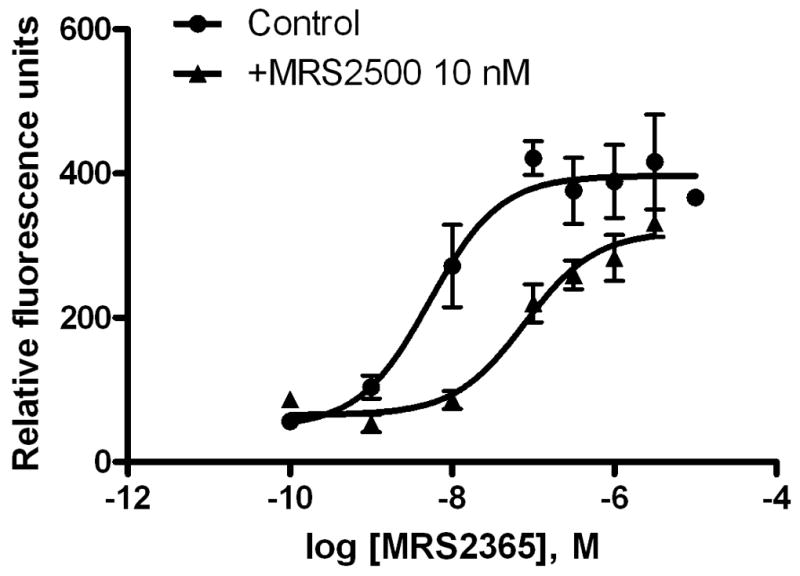

A. Intracellular calcium mobilization induced by a selective P2Y1 receptor antagonist MRS2365. The change in calcium was measured by the addition of P2Y1 agonist or antagonist to the dye and the change in calcium was measured by change in intracellular fluorescence. Samples were run in duplicate with a FLIPRTetra (Molecular Devices, CA) at room temperature. Cell fluorescence (excitation = 485 nm; emission = 525 nm) was monitored following exposure to a compound. Increases in intracellular calcium are reported as the maximum fluorescence value after exposure minus the basal fluorescence value before exposure. Results are from 3–5 separate experiments. B. P2Y1 agonist MRS2365-induced ERK1/2 phosphorylation. PC-3 cells were serum-starved for 4 h before incubation with media containing various pharmacological reagents for the appropriate times at 37°C. Reactions were terminated by removing the media and adding 100 μl of RIPA buffer containing proteinase inhibitors. ERK1/2 activity was measured with an ERK1/2 Colorimetric Detection Kit were from Assay Designs (Ann Arbor, MI) according to the manufacture’s instructions. Results are expressed as mean ± SE from three experiments performed in duplicate. #Significantly different from the basal value (P<0.05).
3.4. P2Y1 receptor-mediated activation of ERK1/2
Exposure of PC-3 cells to the P2Y1 agonist MRS 2365 (1 μM) induced phosphorylation of ERK1/2. Maximal activation was observed after approximately 5 min, and the effect then gradually declined to basal level (Fig. 3B).
3.5. Detection of apoptosis induced by P2Y1 receptor activation
3.5.1. Annexin V staining
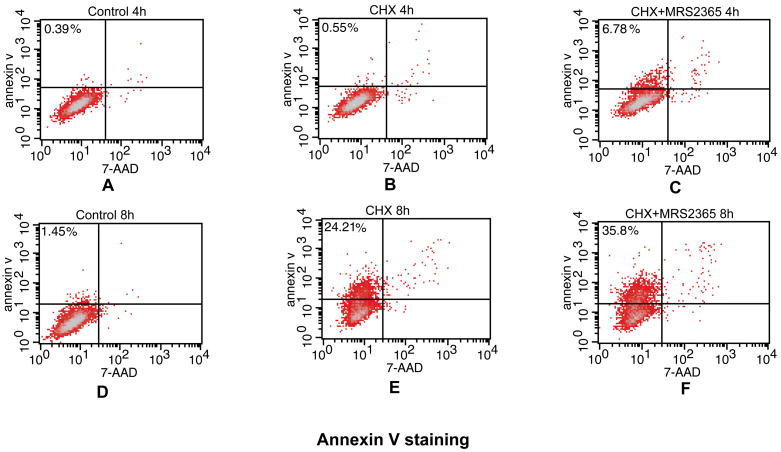
The apoptotic process was first quantified by taking advantage of established flow cytometric methods using annexin V-FITC and 7-AAD [21,22] to measure early (annexin V-FITC-positive cell populations) and late apoptosis (7-AAD-positive cell populations). Fig. 4 shows that there was a significant increase in the number of apoptotic PC-3 cells at 4 h following exposure to CHX alone or in combination with MRS2365. MRS2365 increased early apoptotic PC-3 cells by 6.78%, compared with 0.55% for CHX treatment alone. The proportion of apoptotic cells continued to rise with prolonged treatment time. At 8 h of treatment with MRS2365 and CHX, early apoptotic PC-3 cells increased to 35%, compared with 24% upon treatment with CHX alone (Fig. 4).
Figure 4.
Detection of apoptosis using annexin V-FITC staining. PC-3 cells (5×105) were treated with the P2Y1 agonist MRS2365 in the presence of CHX for 4 h (A–C) or 8 h (D–F). Cells treated both MRS2365 and CHX (C, F) were compared with CHX alone (B, E) and with control cells (A, D). After washing twice with PBS, cells were then re-suspended in 100 μl binding buffer with 5 μl annexin V-FITC and 5 μl 7-AAD and incubated for 15 min. After an additional 200 μl binding buffer was added, cells were analyzed by Becton-Dikinson FACSCalibur flow cytometer (Becton, Dickinson and Company, NJ). Annexin V-FITC was detected in the FL-1 channel (530 nm), while 7-AAD was detected in the FL-3 channel (650 nm). The upper left panels show the number of early apoptotic cells. Data were collected on a log scale and analyzed using CellQuest Pro software.
3.5.3. Caspase-3 activity
The activation of the P2Y1 receptor is know to induce apopotosis in the presence of CHX in astrocytoma cells expressing the recombinant human P2Y1 receptor [7,8]. In the present study, we examined this possibility in PC-3 prostate cancer cells by using the agonist MRS2365 and by measuring the activity of Caspase-3, an early indication of cell apoptosis. Fig. 5A shows that MRS2365 (1 μM) significantly enhanced the Caspase-3 activity. The percentage of Caspase-3 activity in the presence of MRS2365 (141 ± 5%) was significantly different from the control group (100%). P2Y1-specific siRNA significantly inhibited MRS2365-enhanced but not CHX-induced caspase-3 activity (Fig. 5A) (P<0.05, One-way ANOVA with post-hoc test). MRS2365 alone (without CHX) did not induce any increase of caspase-3 activity.
Figure 5.

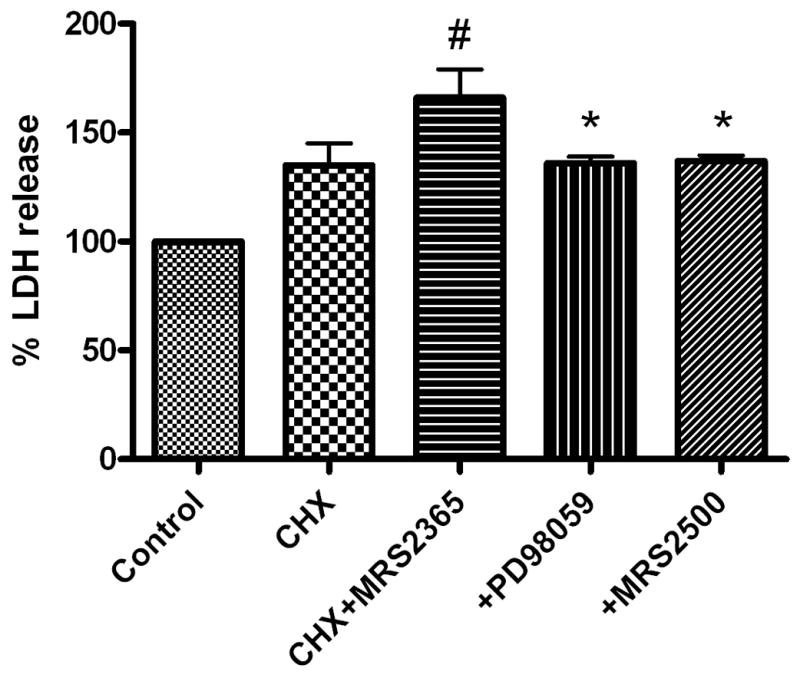

Activation of P2Y1 receptor induces apoptosis in PC-3 cells. A. Caspase-3 activity. PC-3 cells were plated in six-well plates at a density of 2×105 cells per well and cultured to 70% confluence for the experiments. The cells were treated with the P2Y1 receptor agonist MRS2365 (1 μM) in the presence and absence of CHX in the medium for 8 h. Each sample containing 50–200 μg protein was used. The absorbance in each well was measured at 405 nm with a microplate ELISA reader. # Significantly different from CHX + MRS2365 group (P <0.05). B. LDH release. PC-3 cells were cultured in 24-well plate at 37°C overnight with complete medium and 100 μl fresh medium with 1% serum was then added to each well. Increasing concentrations of each agonist, diluted with PBS with calcium and magnesium, and CHX (10 μg/ml) were incubated with the cells at 37°C for 24 h. For the measurement, culture medium was centrifuged and 100 μl supernatant was carefully transferred to corresponding wells of an optically clear 96-well flat bottom microplate in triplicate. LDH release was measured using the Cytotoxicity Detection kit (Roche Applied Science, Indianapolis, IN) following the manufacturer’s instructions. The absorbance of the samples was measured at 490 nm using a SpectraMax5 Microplate reader (Molecular Devices, Sunnyvale, CA). Cell death was assessed based on release of LDH activity. # Significantly different from control (P <0.05). * Significantly different from CHX + MRS2365 group (P <0.05). C. Effect of P2Y1 receptor siRNA on MRS2365-induced cell death. Predesigned siRNAs against human P2Y1 receptors were used. Cells were transfected at about 50–60% confluency by addition of siRNA (1 μM) together with Lipofectamine 2000 transfection reagent as instructed by the manufacturer. Cells were split to 6-well plates 24 h after transfection and incubated for an additional 48 h. 24 h after the second inucubation began, agonist and/or CHX was introduced. LDH release was measured at the end of the 48 h incubation, i.e. after 24 h of agonist exposure. # Significantly different from CHX + MRS2365 group (P <0.05).
3.5.3. LDH release in PC-3 prostate cancer cells
The ability of P2Y1 receptor activation to induce cell death was further examined in an assay of LDH release. The P2Y1 selective agonist MRS2365 (1 μM) induced release of LDH in PC-3 cells in the presence of CHX by 166 ± 13% of basal level, which was significantly higher than the control group (100 %) or the CHX group (135 ± 9.6 %) (P<0.05, One-way ANOVA with post-hoc test). The effect of MRS2365 was blocked by a selective noncompetitive inhibitor of the MAP kinase pathway, PD 98059, and by a selective antagonist of P2Y1 receptors, MRS2500 (Fig. 5B).
3.6. P2Y1 receptor siRNA inhibits LDH release
In a separate set of experiments, specific siRNA for the human P2Y1 receptor was applied to the assay of LDH release. It was demonstrated that the P2Y1 siRNA did not affect CHX-induced release. The percentage release of LDH in the presence of P2Y1 siRNA (93 ± 6.2%) was shown to be not significantly different from the control group (100 ± 5.1 %) (P>0.05). However, the P2Y1 siRNA significantly reduced release of LDH induced by the P2Y1 agonist MRS2365. The percentages of MRS2365-induced LDH release in the presence and absence of P2Y1 siRNA were 117 ± 6 and 146 ± 10, respectively, which were significantly different (P<0.05) (Fig. 5C).
3.7. Activation of the P2Y1 receptor inhibits proliferation of PC-3 prostate cancer cells
The potential anti-proliferative effect of P2Y1 receptor activation was explored with a MTT assay. Fig. 6 shows that the selective P2Y1 agonist MRS2365 alone (1 μM) inhibited cell growth. The cell numbers in the MRS2365-treated group at both 48 h and 72 h were significantly lower than in control group (P<0.05, paired test). The specific siRNA for human P2Y1 receptor significantly attenuated the effect of MRS2365 (Fig. 6).
Figure 6.

Measurement of cell proliferation with the MTT assay. PC-3 cells were seeded in 96-well plates about 1×104 cells per well in a volume of 100 μl of cell culture medium with 10% serum per well and cultured at 37°C overnight. The P2Y1-receptor agonist MRS2365 (1 μM) was incubated with the cells at 37°C in a CO2 incubator for 24, 48, and 72 h. MRS2365 (1 μM) or vehicle was applied to the medium every 12 h. Cell viability was measured using a MTT Toxicology Assay Kit (Sigma-Aldrich, St. Louis, MO) following the manufacturer’s protocol. The P2Y1 specific siRNA was transfected using Lipofectamine 2000 transfection reagent as instructed by the manufacturer. The absorbance was quantified by measuring at 570 nm with a SpectraMax5 Microplate reader (Molecular Devices, Sunnyvale, CA) and was transformed into cell numbers based on a standard curve measured with various numbers of cells.
# Significantly different from control (P <0.05).
4. Discussion
In the present study, the presence of a functional P2Y1 receptor in PC-3 prostate cancer cells, a model of androgen-independent cancer, has been demonstrated both genetically and pharmacologically. The abundant expression of the P2Y1 receptor is consistent with a previous report in primary prostate cells [16].
Apoptosis induced by activation of the P2Y1 receptor endogenously expressed in PC-3 prostate cancer cells demonstrated in the present study is consistent with a previously reported functional effect of P2Y1 receptor activation in 1321N1 astrocytoma cells expressing the recombinant human P2Y1 receptor [7,8]. In 1321N1 astrocytoma cells, P2Y1 receptor-mediated apoptosis has been correlated to ERK1/2 activation [7], and the present study further examined the potential involvement of MAP kinase signaling in prostate cancer cells. Indeed, the selective P2Y1 agonist MRS2365 was able to induce ERK1/2 phosphorylation, and a MAP kinase inhibitor, PD98059, significantly blocked cell apoptosis induced by P2Y1 receptor activation, suggesting a crucial role of ERK1/2 signaling in prostate cancer cells.
In addition to the P2Y1 receptor demonstrated in the present study, the roles of several other P2Y subtypes in growth and death of various types of cells have also been reported. P2Y13 receptor has been reported to mediate ADP-induced apoptosis in pancreatic beta cells [11]. Activation of the P2Y2 receptor has been reported to inhibit apoptosis [13]. P2Y2 was identified as a critical sensor of nucleotides released by apoptotic cells [24]. Activation of P2Y6, P2Y11 and P2Y12 receptors, but not the P2Y4 receptor, was reported to protect cells against TNF-α-induced apoptosis [8,12,14].
In addition to the induction of apoptosis, the P2Y1 receptor has also been shown to mediate inhibitory effects on cell proliferation by several P2Y receptor agonists. The anti-proliferative effect of the P2Y1 receptor was first demonstrated in 1321N1 astrocytoma cells stably expressing the recombinant human P2Y1 receptor [7]. In that study, 300 nM 2-MeSADP significantly diminished carbachol-induced cell proliferation. In a later study, White et al. found that ATP also played an anti-proliferative role in melanoma cells and suggested that its effect was through activation of the P2Y1 receptor [9]. The same authors also found that ATP treatment reduces volume and weight of tumors in an in vivo melanoma model [10]. Importantly, melanoma cells overexpress two extracellular nucleotidases, namely autotaxin (NPP2) and CD39 (NTPD1) [25,26]. The first enzyme, besides showing a lysophospholipase activity, acts as an ecto-nucleotide pyrophosphatase/phosphodiesterase that catalyzes the direct breakdown of extracellular ATP to AMP; the second one, instead, sequentially catalyzes the breakdown of ATP to ADP and then of ADP to AMP. The action of autotaxin and, even more pronouncedly, that of CD39 result in the depletion of the extracellular levels of ATP and ADP, with a consequent reduced stimulation of the P2Y1 receptor. The finding from the present study that activation of the P2Y1 receptor by a selective P2Y1 receptor agonist MRS2365 inhibits proliferation of PC-3 cell is in line with those earlier results.
Adenine nucleotides are known to have many functions in addition to their effect on P2Y1 receptors [3]. It has been reported ATP may inhibit proliferation in various cells via P2X receptor activation, in addition to its action at the P2Y1 receptor demonstrated in the present study [4,22,27,28]. It has also been reported that ATP and ADP exert tissue-protective and proliferative effects via activation of the P2Y1 receptor [15,29–31]. However, it should be noted that nucleotides such as ATP and ADP often have effects on multiple P2X or P2Y receptor subtypes, depending on the expression profile of those receptors. Unlike those previous studies, the present study demonstrated a high expression level of the P2Y1 receptor both functionally and genetically. Furthermore, the role of the P2Y1 receptor in both cell death and cell proliferation was characterized using a selective P2Y1 agonist MRS2365. Thus, the present study convincingly demonstrated the involvement of the P2Y1 receptor in cell death and growth of human prostatic carcinoma PC-3 cells, suggesting beneficial agonist effects. P2 agonists have already been suggested as having a beneficial effect in models of melanoma, in which the P2Y1 receptor is highly expressed [10]. Other potential therapeutic applications under consideration for P2Y1 receptor ligands include: selective agonists as antidiabetic agents [32] or selective antagonists as antithrombotic agents [33]. P2Y1 agonists that are dinucleotides with enhanced in vivo stability have been reported [32].
In summary, the dual pro-apoptotic and anti-proliferative effects of P2Y1 receptor activation demonstrated in the present study indicate that the P2Y1 receptor might be an attractive target for the treatment of prostate cancer.
Acknowledgments
Supported by the NIDDK Intramural Research Program, National Institutes of Health, Bethesda, MD, USA, National Natural Science Foundation of China (No: 30940072) and Nanfang Hospital, Southern Medical University, Guangzhou, China. The authors thank Dr. Yafang Hu (Children’s National Medical Center, Washington, DC) for assistance in Western blotting and confocal microscopy experiments.
Abbreviations
- 7-AAD
7-aminoactinomycin D
- CHX
cycloheximide
- ERK
extracellular receptor-activated kinase
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- LDH
lactate dehydrogenase
- MAP kinase
mitogen-activated protein kinase
- MRS2179
N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate
- MRS2365
(N)-methanocarba-2′-deoxy-2-methylthio-adenosine-5′-diphosphate
- MRS2500
2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate
- 2-MeSADP
2-methylthioadenosine 5′-diphosphate
- MTT
3-(4,5-dimethylethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PBS
phosphate-buffered saline
- TNF-α
tumor necrosis factor-alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10:47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- 2.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 3.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346–58. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson KA, Boeynaems JM. P2Y nucleotide receptors: promise of therapeutic applications. Drug Discov Today. 2010;15:570–8. doi: 10.1016/j.drudis.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao ZG, Ding Y, Jacobson KA. P2Y13 receptor is responsible for ADP-mediated degranulation in RBL-2H3 rat mast cells. Pharmacol Res. 2010;62:500–5. doi: 10.1016/j.phrs.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sellers LA, Simon J, Lundahl TS, Cousens DJ, Humphrey PP, Barnard EA. Adenosine nucleotides acting at the human P2Y1 receptor stimulate mitogen-activated protein kinases and induce apoptosis. J Biol Chem. 2001;276:16379–90. doi: 10.1074/jbc.M006617200. [DOI] [PubMed] [Google Scholar]
- 8.Mamedova LK, Gao ZG, Jacobson KA. Regulation of death and survival in astrocytes by ADP activating P2Y1 and P2Y12 receptors. Biochem Pharmacol. 2006;72:1031–41. doi: 10.1016/j.bcp.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White N, Ryten M, Clayton E, Butler P, Burnstock G. P2Y purinergic receptors regulate the growth of human melanomas. Cancer Lett. 2005;224:81–91. doi: 10.1016/j.canlet.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 10.White N, Knight GE, Butler PE, Burnstock G. An in vivo model of melanoma: treatment with ATP. Purinergic Signal. 2009;5:327–33. doi: 10.1007/s11302-009-9156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan C, Salehi A, Svensson S, Olde B, Erlinge D. ADP receptor P2Y13 induce apoptosis in pancreatic beta-cells. Cell Mol Life Sci. 2010;67:445–53. doi: 10.1007/s00018-009-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SG, Soltysiak KA, Gao ZG, Chang TS, Chung E, Jacobson KA. Tumor necrosis factor alpha-induced apoptosis in astrocytes is prevented by the activation of P2Y6, but not P2Y4 nucleotide receptors. Biochem Pharmacol. 2003;65:923–31. doi: 10.1016/s0006-2952(02)01614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arthur DB, Georgi S, Akassoglou K, Insel PA. Inhibition of apoptosis by P2Y2 receptor activation: novel pathways for neuronal survival. J Neurosci. 2006;26:3798–804. doi: 10.1523/JNEUROSCI.5338-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaughan KR, Stokes L, Prince LR, Marriott HM, Meis S, Kassack MU, Bingle CD, Sabroe I, Surprenant A, Whyte MK. Inhibition of neutrophil apoptosis by ATP is mediated by the P2Y11 receptor. J Immunol. 2007;179:8544–53. doi: 10.4049/jimmunol.179.12.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quintas C, Fraga S, Gonçalves J, Queiroz G. Opposite Modulation of Astroglial Proliferation by ADPβS and 2-MeSADP: Mechanisms Involved. Neuroscience. 2011 Mar 15; doi: 10.1016/j.neuroscience.2011.03.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Janssens R, Communi D, Pirotton S, Samson M, Parmentier M, Boeynaems JM. Biochem Biophys Res Commun. Cloning and tissue distribution of the human P2Y1 receptor. Biochem Biophys Res Commun. 1996;221:588–93. doi: 10.1006/bbrc.1996.0640. [DOI] [PubMed] [Google Scholar]
- 17.Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK. Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analogue. J Pharm Exp Therap. 2004;311:1038–1043. doi: 10.1124/jpet.104.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HS, Ohno M, Xu B, Kim HO, Choi Y, Ji XD, Maddileti S, Marquez VE, Harden TK, Jacobson KA. 2-Substitution of adenine nucleotide analogues containing a bicyclo [3.1. 0]hexane ring system locked in a Northern conformation: Enhanced potency as P2Y1 receptor antagonists. J Med Chem. 2003;46:4974–4987. doi: 10.1021/jm030127+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2( Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Gao ZG, Ding Y, Jacobson KA. UDP-glucose acting at P2Y14 receptors is a mediator of mast cell degranulation. Biochem Pharmacol. 2010;79:873–9. doi: 10.1016/j.bcp.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 22.Schmid I, Krall WJ, Uittenbogaart CH, Braun J, Giorgi JV. Dead cell discrimination with 7-amino-actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry. 1992;13:204–208. doi: 10.1002/cyto.990130216. [DOI] [PubMed] [Google Scholar]
- 23.Janssens R, Boeynaems JM. Effects of extracellular nucleotides and nucleosides on prostate carcinoma cells. Br J Pharmacol. 2001;132:536–46. doi: 10.1038/sj.bjp.0703833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–6. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dzhandzhugazyan K, Kirkin A, thor Straten P, Zeuthen J. Ecto-ATP diphosphohydrolase/CD39 is overexpressed in differentiated human melanomas. FEBS Lett. 1998;430:227–30. doi: 10.1016/s0014-5793(98)00603-6. [DOI] [PubMed] [Google Scholar]
- 26.Jankowski M. Autotaxin: its role in biology of melanoma cells and as a pharmacological target. Enzyme Res. 2011;2011:ID194857. doi: 10.4061/2011/194857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng L, Sun X, Csizmadia E, Han L, Bian S, Murakami T, Wang X, Robson SC, Wu Y. Vascular CD39/ENTPD1 Directly Promotes Tumor Cell Growth by Scavenging Extracellular Adenosine Triphosphate. Neoplasia. 2011;13:206–16. doi: 10.1593/neo.101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shabbir M, Burnstock G. Purinergic receptor-mediated effects of adenosine 5′-triphosphate in urological malignant diseases. Int J Urol. 2009;16:143–50. doi: 10.1111/j.1442-2042.2008.02207.x. [DOI] [PubMed] [Google Scholar]
- 29.Shinozaki Y, Koizumi S, Ishida S, Sawada J, Ohno Y, Inoue K. Cytoprotection against oxidative stress-induced damage of astrocytes by extracellular ATP via P2Y1 receptors. Glia. 2005;49:288–300. doi: 10.1002/glia.20118. [DOI] [PubMed] [Google Scholar]
- 30.Battista AG, Ricatti MJ, Pafundo DE, Gautier MA, Faillace MP. Extracellular ADP regulates lesion-induced in vivo cell proliferation and death in the zebrafish retina. J Neurochem. 2009;111:600–13. doi: 10.1111/j.1471-4159.2009.06352.x. [DOI] [PubMed] [Google Scholar]
- 31.Franke H, Sauer C, Rudolph C, Krügel U, Hengstler JG, Illes P. P2 receptor-mediated stimulation of the PI3-K/Akt-pathway in vivo. Glia. 2009;57:1031–45. doi: 10.1002/glia.20827. [DOI] [PubMed] [Google Scholar]
- 32.Eliahu S, Barr HM, Camden J, Weisman GA, Fischer B. A novel insulin secretagogue based on a dinucleoside polyphosphate scaffold. J Med Chem. 2010;53:2472–81. doi: 10.1021/jm901621h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Léon C, Hechler B, Freund M, Eckly A, Vial C, Ohlmann P, Dierich A, LeMeur M, Cazenave JP, Gachet C. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y1 receptor-null mice. J Clin Invest. 1999;104:1731–7. doi: 10.1172/JCI8399. [DOI] [PMC free article] [PubMed] [Google Scholar]