Table 2.
Tertiary ether substrates in stereoselective cyclization reactions.[a]
| Entry | Substrate[b] | Product | t [h] | d.r.[c] | Yield [%][d] |
|---|---|---|---|---|---|
| 1 |
 23 |
 24 |
2.5 | 100:0 | 79 |
| 2 |
 25 |
 26 |
2 | 15.7:1 | 95 |
| 3 |
 27 |
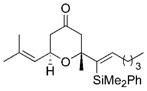 28 |
0.5 | 100:0 | 76 |
| 4 |
 29 |
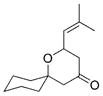 30 |
0.3 | – | 88 |
| 5[e] |
 31 |
 32 |
5 | 100:0 | 81 |
| 6[f] |
 33 |
– | – | – | – |
Typical procedure: a solution of the substrate and 2,6-dichloropyridine in 1,2-dichloroethane was treated with DDQ and stirred for the indicated time period.
See the Supporting Information for details on substrate synthesis.
d.r. = diastereomer ratio normalized to 100.
Yields refer to isolated, purified material.
Reaction was conducted at −30°C.
no reaction occurred.
