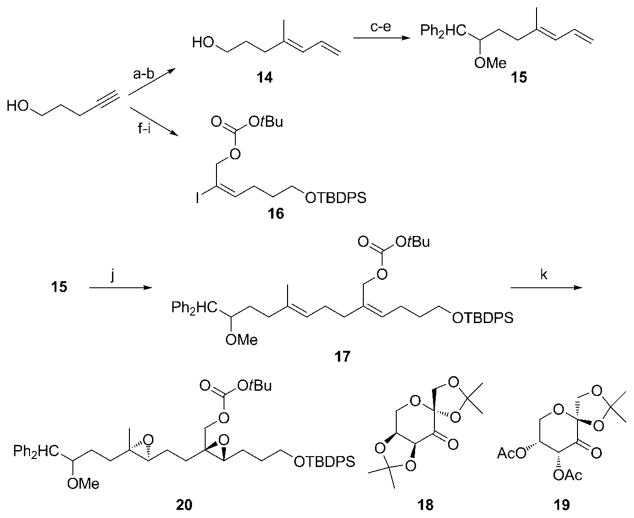
Scheme 3.
Synthesis of the cascade cyclization substrate. Reagents and conditions: a) Me3Al, Cp2ZrCl2, H2O, DCE, then I2, 94%; b) CH2=CHMgBr, [Pd(PPh3)4], PhMe, 91%; c) Oxalyl chloride, DMSO, CH2Cl2, Et3N, −78 °C; d) Ph2CH2, nBuLi, THF, 82% (two steps); e) NaH, DMF, then MeI, 96%; f) TBDPSCl, imidazole, DMF, 100%; g) nBuLi, THF, then (CH2O)n, 94%; h) Bu3SnH, [Pd(PPh3)4], C6H6, then I2, CH2Cl2,83%; i) (Boc)2O, N-methylimidazole, PhMe, 99%; j) 9-BBN dimer, THF, then 16, [Pd(PtBu3)2], K3PO4, H2O, PhMe, 74%; k) Oxone, 18, K2CO3, Bu4NHSO4, CH3CN, H2O, then 19, 82 %. 9-BBN = 9-borabicyclo[3.3.1] nonane, Boc =tert-butyloxycarbonyl, DMF =N,N′-dimethylformamide, DMSO=dimethyl sulfoxide, TBDPS =tert-butyldiphenylsilyl, THF =tetrahydrofuran.