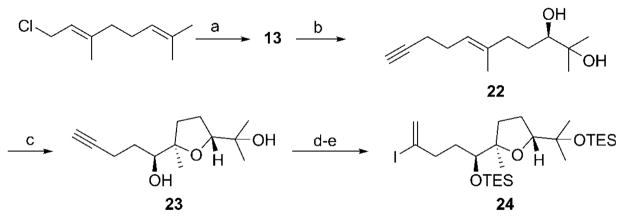
Scheme 5.
Synthesis of the right-hand fragment. Reagents and conditions: a) 1-Trimethylsilylpropyne, nBuLi, THF, −78°C, then Bu4NF, 88%; b) AD-Mix β, CH3SO2NH2, tBuOH, H2O, 53 %; c) 18, Oxone, K2CO3, Bu4NHSO4, CH3CN, H2O, then Py·CSA, 83%, d. r. = 13:1; d) TESCl, imidazole, DMAP, DMF, 89%; e) Et3SiH, [CpRu-(NCCH3)3]PF6, CH2Cl2, then I2, 2,6-lutidine, 82%. Cp = cyclopentadienyl, DMAP =4-dimethylaminopyridine, Py·CSA=pyridinium camphorsulfonate, TES =triethylsilyl.