Fig. 2.
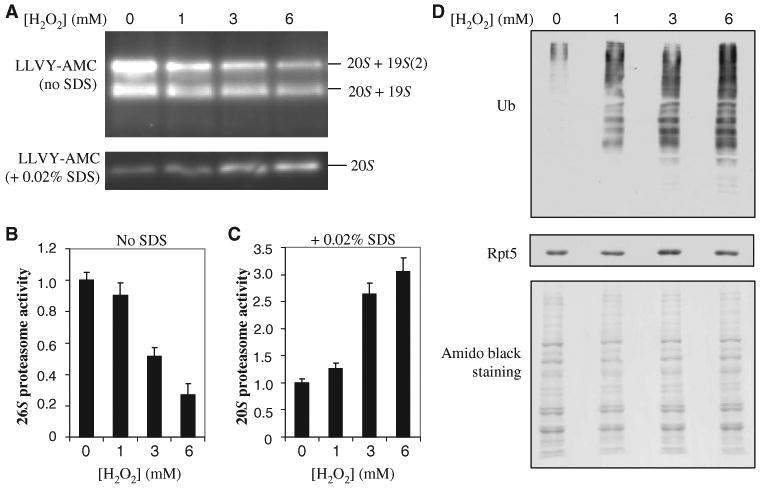
Effects of H2O2-induced dissociation of the 20S core from the 19S particle. (A) Proteasome activities after oxidative stress. Lysates from cells treated with the indicated concentrations of H2O2 were resolved by native gel electrophoresis. Chymotrypsin-like activity was measured with a native gel overlay assay with the fluorogenic peptide substrate SUC-LLVY-AMC in the absence or presence of 0.02% SDS to assay the 26S and 20S proteasomes, respectively. (B and C) Quantitation of the proteasome activities detected in (A) with a Fuji LAS400 imager for (B) 26S proteasomes and (C) 20S proteasomes. (D) Detection of total ubiquitin conjugates after H2O2-induced stress by Western blotting analysis with an antibody against ubiquitin. Equivalent loading was determined by analysis of Western blots with an antibody against Rpt5 and by staining of the membrane with amido black. Data are from three experiments.
