Fig. 3.
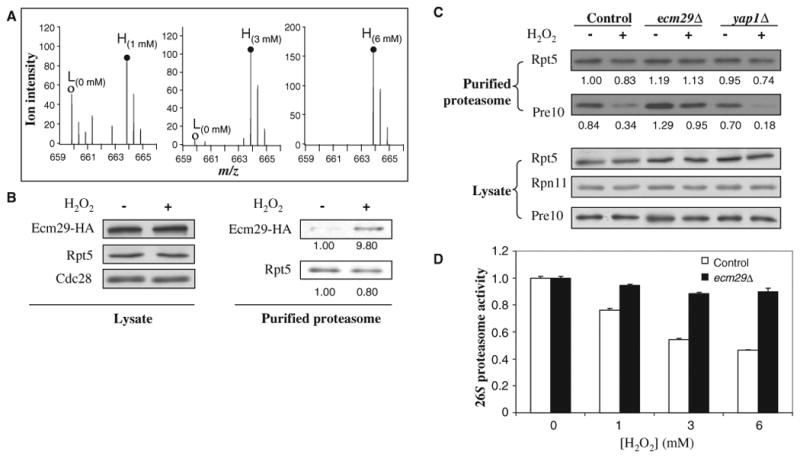
Ecm29 recruitment and its effect on the dissociation of the yeast 26S proteasome. (A) Enrichment of Ecm29 in purified 26S proteasome complexes as determined by SILAC-MS analysis. MS spectra of one representative Ecm29 tryptic peptide pair [MH22+ 659.86, VNNASINTSATVK versus MH22+ 663.86, VNNASINTSATVK (label: 13C615N2)]. (○), untreated (L); (●), treated (H). The SILAC ratios (H/L) of Ecm29 were 1.6, 17.3, and 43.7 when the cells cultured in heavy medium were treated with H2O2 (1, 3, and 6 mM) in comparison to the untreated cells cultured in light medium. Abbreviations for the amino acids are as follows: A, Ala; I, Ile; K, Lys; N, Asn; S, Ser; T, Thr; and V, Val. (B) Validation of H2O2-triggered enrichment of Ecm29 in the purified 19S particle by quantitative Western blotting. The abundances of Ecm29-HA proteins in lysates and bound to purified proteasomes from untreated and treated cells were measured by Western blotting with antibodies against the HA tag. Cells were treated with H2O2 (3 mM). (C) Roles of Ecm29 and Yap1 in the oxidative stress–induced disassembly of the 26S proteasome as determined by quantitative Western blotting. Wild-type cells and ecm29Δ or yap1Δ cells containing Rpn11-TAP were untreated or treated with H2O2 (3 mM) for 30 min. The purified proteasome complexes were analyzed by quantitative Western blotting with antibodies against Rpt5 (a 19S subunit) and Pre10 (a 20S subunit) and an Odyssey Infrared Imaging System. The number below each band represents its intensity. (D) Effect of ECM29 deletion on the activity of the 26S proteasome. Wild-type and ecm29Δ cells grown to mid-log phase were left untreated or treated with H2O2 (1, 3, or 6 mM) for 30 min, and cell lysates were analyzed for chymotrypsin-like proteasome activity. Data are from three experiments.
