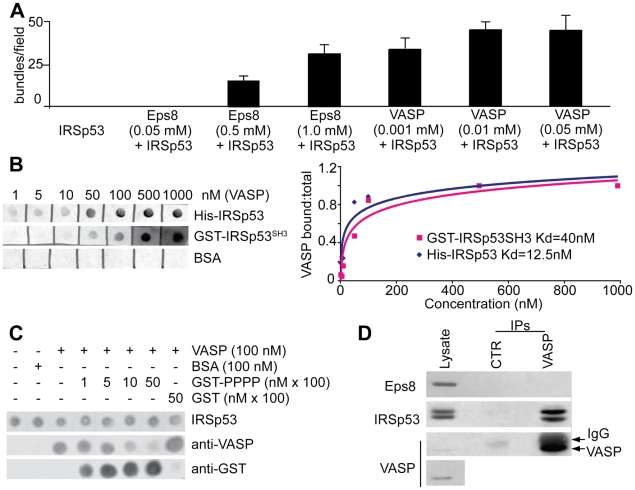
Figure 2. VASP synergizes with IRSp53 in bundling actin filaments and competes with Eps8 for IRSp53 binding.
a. Isolated VASP and Eps8 bundle actin filaments with low efficiency, which is enhanced by their association with IRSp53. The bundling efficiency was determined by measuring the number of bundles/field obtained in fluorescence microscopy-based F-actin-bundling assays as described and shown in Fig. S1A–B. At least 10 fields per experiment performed in triplicates were scored. Data are the mean ± s.e.d. b. Measurement of IRSp53 and VASP interaction. Equal amounts (10 pmoles) of His-IRSp53, GST-IRSp53-SH3 or BSA were spotted onto nitrocellulose and incubated with increasing concentrations of purified VASP. The nitrocellulose filter was then subjected to WB analysis using anti-VASP antibody (Ab). The fraction of VASP bound was plotted against the concentrations of total VASP. An apparent dissociation constant was calculated using standard procedure as described in [12]. c. The proline rich region of Eps8 (PPP) competes with VASP for binding to IRSp53. Equal amounts (10 pmoles) of His-IRSp53 spotted onto nitrocellulose and incubated with purified 100 nM VASP or BSA as control, in the absence or the presence of increasing amounts of the proline-rich region of Eps8 (GST-PPP) or GST. The filters were immunoblotted with the indicated abs. d. VASP forms a complex with IRSp53 in-vivo. Lysates (1 mg) of HeLa cells were immunoprecipitated with anti-VASP or with control abs. Lysates (20 µg) and immunoprecipitates (IP) were immunoblotted with the indicated abs. The bottom panel is a longer exposure to visualize endogenous levels of VASP.