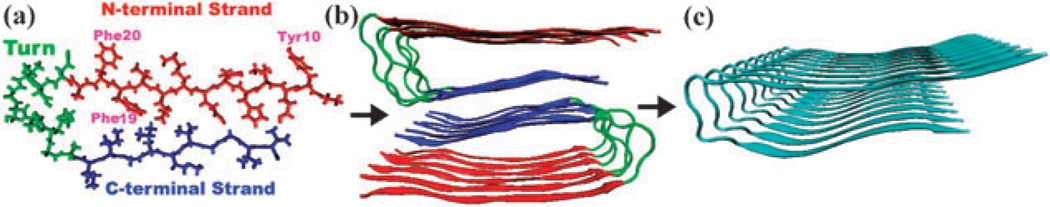
Fig. 4.
(a) Aβ1–40 sequence and the structure of a single Aβ9–40 peptide, with N-terminal strand (red), C-terminal strand (blue), and turn region (green). It has three aromatic side chains, Tyr10, Phe19, Phe20, all located on the N-terminal. (b) Model structure of amyloid fibril made of twelve Aβ9–40 molecules. Red and blue indicate two parallel β-sheets. (c) The model periodic fibril structure made of nine peptides used in the UV spectroscopy simulations.