Abstract
PURPOSE
The object of this study was to investigate the effect of UV irradiation (by a general commercial UV sterilizer) on anodized titanium surface. Surface characteristics and cellular responses were compared between anodized titanium discs and UV irradiated anodized titanium discs.
MATERIALS AND METHODS
Titanium discs were anodized and divided into the following groups: Group 1, anodized (control), and Group 2, anodized and UV irradiated for 24 hours. The surface characteristics including contact angle, roughness, phase of oxide layer, and chemical elemental composition were inspected. The osteoblast-like human osteogenic sarcoma (HOS) cells were cultured on control and test group discs. Initial cellular attachment, MTS-based cell proliferation assay, and ALP synthesis level were compared between the two groups for the evaluation of cellular response.
RESULTS
After UV irradiation, the contact angle decreased significantly (P<.001). The surface roughness and phase of oxide layer did not show definite changes, but carbon showed a considerable decrease after UV irradiation. Initial cell attachment was increased in test group (P=.004). Cells cultured on test group samples proliferated more actively (P=.009 at day 2, 5, and 7) and the ALP synthesis also increased in cells cultured on the test group (P=.016 at day 3, P=.009 at day 7 and 14).
CONCLUSION
UV irradiation induced enhanced wettability, and increased initial cellular responses of HOS cells on anodized titanium surface.
Keywords: UV light, Anodization, Wettability, Cell attachment, Proliferation, Differentiation
INTRODUCTION
Since Brånemark et al.1 introduced the concept of osseointegration, titanium has been proven to have excellent biocompatibility, and is now widely used in oral rehabilitation.
Tissue reactions to dental implant are determined by the implant surface quality.2 After implantation, an implant surface is conditioned by blood and tissue fluid containing proteins, ions, lipids, and sugars,3 Surface composition, roughness, topography, and surface energy are factors that affect this process.4 The wettability and surface charge are important in protein adsorption to titanium dioxide.5 Wang et al. discovered that the wettability of titanium dioxide increased after Ultraviolet (UV) light irradiation, and the wettability change occurred on both anatase and rutile TiO2 surfaces of polycrystals or single crystals.6 UV irradiation seems to create surface oxygen vacancies at bridging site, and this leads to conversion of relevant Ti4+ sites to Ti3+ sites.7 After conversion to Ti3+, titanium dioxide surface is suitable for dissociative water adsorption.8-10 Ti3+ defects can be created by Ar+ bombardment, electron beam exposure, high-temperature vacuum annealing, and low-energy ultraviolet photons irradiation. The largest Ti4+ to Ti3+ conversion occurs by UV irradiation.11
In air at room temperature, the titanium surface is covered spontaneously by an oxide layer with its range from 1.5 to 10 nm in thickness.12 It has been reported that the oxide layer has a low level of electronic conductivity,13 great thermodynamic stability14 and low ion-formation tendency in aqueous environment.15 Anodic oxidation is an electrochemical treatment that forms thick, rough, porous titanium oxide layer on titanium surface.16 Rabbit studies revealed that titanium implants treated by anodic oxidation shows better bone response than machined titanium implants.16,17 Among various implant surface treating methods, anodic oxidation is the popular one, clinically proven and widely used.18 The hydrophilicity of anodized titanium surface can be improved by UV irradiation, because TiO2 crystals (Anatase, Rutile) which react to UV irradiation are increased by anodic oxidation.19 The elevated hydrophilicity of anodized tiatanium surface may lead to the promotion of cellular response after implantation.
The aim of present study is to investigate the effect of UV irradiation (by commercial UV sterilizer) on anodized titanium surface. The surface characteristics were compared between anodized titanium discs and UV irradiated anodized titanium discs, and cellular responses including initial cellular attachment, proliferation and differentiation were also evaluated and compared.
MATERIALS AND METHODS
1. Titanium disc preparation
Titanium discs with dimensions of 25 mm diameter and 1 mm thickness were made of commercially pure titanium (Warrentec Co., Seoul, Korea). For XPS inspection, 10 mm×10 mm×1 mm square shaped discs were used. Titanium discs were anodized at 300 V in an aqueous electrolytic solution of 0.02 mol calcium glycerophosphate and 0.15 mol calcium acetate monohydrate for 3 minutes. After anodization, discs were rinsed with distilled water, dried and sterilized in ethylene oxide gas. The discs were divided into following two groups
·Group 1 (control): anodized under 300 V
·Group 2 (test): anodized under 300 V, and UV irradiated for 24 hours just before experiment
UV illumination was done by UV sterilizer (DS-701, Daeshin electric industry, Incheon, Korea) with 15 W bactericidal UV lamp.
2. Contact angle measurement
Using a video contact angle analyzer (General type Phoenix 150, SEO, Seoul, Korea), static constant angle of water were measured by the sessile drop method. On dripping water (3.5 µl) on discs, images were taken immediately, and the contact angles were measured and recorded by the Image XP program (General type Phoenix 150, SEO, Seoul, Korea). 6 discs for each group were examined and 5 points in each disc (total 30 points for each group) were randomly chosen for contact angle measurement.
3. Surface roughness measurement
The surface roughness of control and test group was inspected by a confocal laser scanning microscope (LSM 5 Pascal, Carl-Zeiss, Oberkochen, Germany). One anodized disc was cut into two pieces using cutting machine (EXAKT 300, EXAKT Advanced Technologies Gmbh, Norderstedt, Germany). One piece was contained in dark chamber as control and the other piece was illuminated in UV sterilizer for 24 hours just before testing. Five points in each sample were randomly chosen for scan. Surface area roughness (Sa) values were recorded and compared between the two groups.
4. Surface analysis with XRD and XPS
The phases of oxide layers were analyzed by X-ray diffraction (D8 ADVANCE, Bruker, Karlsruhe, Germany). The chemical elemental compositions were examined by X-ray photoelectron spectroscopy (AXIS-HSi, Kratos Analytical Ltd., Manchester, England).
5. Cell culture
Osteoblast-like human osteogenic sarcoma cells (HOS, KCLB, Seoul, Korea) were cultured in RPMI medium (GIBCO, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS, GIBCO, CA, USA), 0.5% penicillin (10,000 U), and streptomycin (10,000 µg/ml), under humidified atmosphere of 95% air and 5% carbon dioxide at 37℃. One titanium disc was contained in one culture dish (35 mm in diameter).
6. Cell attachment assay
Cells were seeded onto each disc at a density of 5×105 cells/cm2. After 2 hours of incubation, cells were rinsed with phosphate-buffered saline (PBS, GIBCO, CA, USA), and fixed with 10% formaline solution (Sigma, Steinheim, Germany) for 15 minutes. 1.0% toluidine blue solution (Sigma, Steinheim, Germany) was applied and rinsed three times with distilled water. After cell lysis induced by application of 2% sodium dodecyl sulfate (SDS, Sigma, Steinheim, Germany), color changes were measured at 600 nm using a 96-well plate spectrophotometer (Powerwave 340, Bio-Tek, Winooski, VT, USA). For cell attachment assay, 6 discs per group (n = 6) were used.
7. Cell proliferation assay
EZ-Cytox cell viability assay kit (Daeil lab service Co, Seoul, Korea) was used as prescribed in manufacturer's manual in measuring cell proliferatation on discs. Cells were seeded onto each disc at a density of 5×104 cells/cm2. The optical densities (ODs) of the samples were measured using a spectrophotometer at 460 nm, after 2, 5, and 7 days of culture. Total 30 discs (5 discs per group in each culture) were used.
8. Alkaline phosphatase (ALP) activity assay
Cells were seeded on each disc at a density of 5×104 cells/cm2. After 3, 7, and 14 days of culture, cells were lysed with 25 mM glycine buffer (0.5% Triton x-100). p-nitrophenol phosphate (pNPP, Sigma, Steinheim, Germany) was added to culture dish and incubated for 30 min. at 37℃. 2 N NaOH solution was used as a stop solution. ALP activity of cultured cells was tested by a colorimetry-based assay utilizing the conversion of colorless to colored p-nitrophenol. The absorbance of the samples was measured spectrometrically at 405 nm. Total 30 discs (5 discs per group in each culture) were used.
9. Statistical analysis
All data analysis was performed by Mann-Whitney U test (PASW statics 17.0 software, SPSS Inc, Chicago, IL, USA).
RESULTS
1. Contact angle analysis
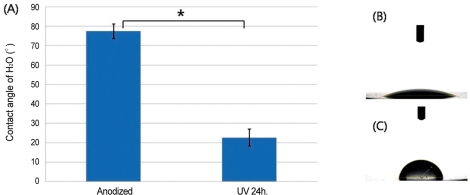
The contact angle measurements showed that UV irradiated anodized titanium was significantly more hydrophilic than anodized titanium (P<.001). The mean values of contact angle measurements were 22.69 ± 4.35° (Mean ± SD) for test group and 77.43 ± 3.80° for control group (Fig. 1).
Fig. 1.
A: Mean contact angles (± SD) of H2O on the discs, B: Image of H2O droplet on UV irradiated anodized titanium disc, C: Image of H2O droplet on anodized titanium disc (*: P<.001).
2. Surface roughness
The surface area roughness (Sa) values of two groups did not show significant difference. The Sa values were 0.833 ± 0.032 µm (Mean ± SD) for control group and 0.854 ± 0.026 µm for test group.
3. Phase of oxide layer and chemical composition
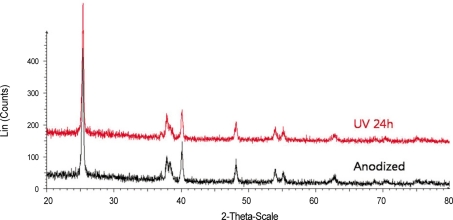
The phase of oxide layer of anodized titanium did not change after UV irradiation for 24 hours. XRD result indicated the intensity and position of peaks were almost identical between group 1 and 2 (Fig. 2).
Fig. 2.
XRD patterns of control and test sample.
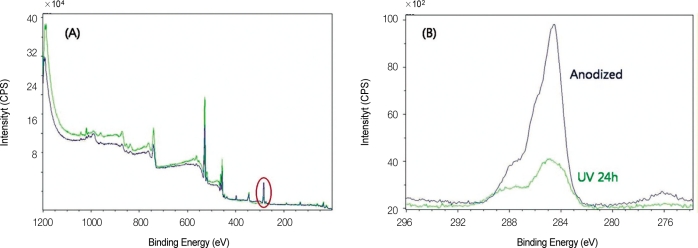
In XPS spectra, C1s peak showed considerable difference (Fig. 3). After UV irradiation, carbon decreased from 42.98% to 17.18% in atomic concentration, and from 30.28% to 10.46% in mass concentration.
Fig. 3.
A: XPS spectrum of anodized titanium and UV irradiated anodized titanium, B: Narrow scan of C1s peak (area in red circle of Fig. 3(A)).
4. Cell attachment
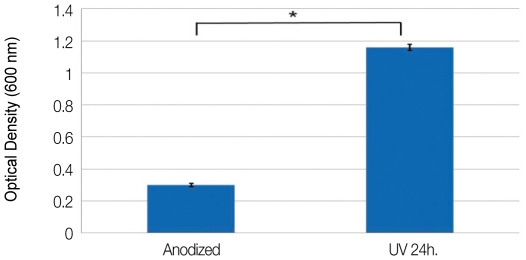
The optical density (OD) value of anodized surfaces was 0.30 ± 0.01, and that of UV irradiated anodized surfaces was 1.16 ± 0.02 (Fig. 4). The difference was statistically significant (P=.004).
Fig. 4.
Optical densities of cellular attachment after 2 hours of incubation (*: P=.004).
5. Cell proliferation
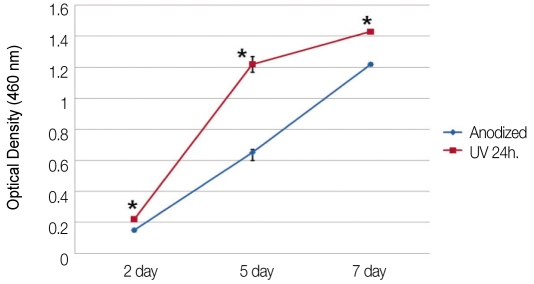
The results of MTS assays are shown in Fig. 5. Cells proliferated more actively on UV irradiated anodized titanium discs. The OD values of UV irradiated anodized surfaces were significantly higher than that of anodized surfaces in all cultures (P=.009 at day 2, 5, and 7).
Fig. 5.
Changes of optical densities indicating the level of cell proliferation (*: P=.009).
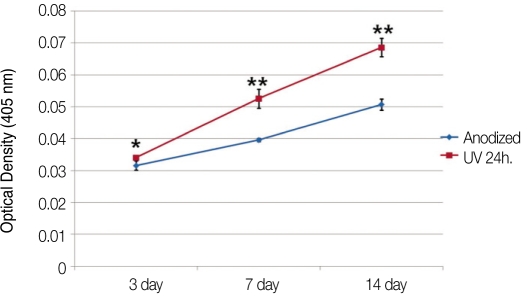
6. Alkaline phosphatase (ALP) synthesis
Fig. 6 shows the result of ALPase synthesis. The OD values of experimental group were significantly higher than the value of control group in all cultures (P=.016 at day 3, P=.009 at day 7 and 14).
Fig. 6.
Changes of optical densities indicating the level of ALP synthesis (*: P=.016, **: P=.009).
DISCUSSION
Surface wettability is largely dependent on surface energy and increased wettability enhances interaction between implant surface and the biologic environment.20 According to Eriksson et al.21, hydrophilic titanium surface has higher surface energy than hydrophobic titanium, and resulted in more rapid cell activation and differentiation. The contact angle of anodized titanium disc was significantly decreased by UV irradiation (P<.001), and the surface energy was elevated. Anodized titanium implant showed significantly higher bone-implant contact ratio and bone volume than machined titanium implant in rabbit study.16 This means that in rabbit tibiae, osteoblastic differentiation and proliferation are more active on anodized titanium surface and also imply that osseointegration is faster and promoted during the same healing period on anodized titanium surface. The initial cellular response to anodized titanium and UV irradiated anodized titanium was inspected and compared in this study. The cellular attachment level after 2 hours of incubation of test group was significantly higher than that of control group (P<.001), and the difference was almost 4 times. The MTS assay for evaluation of cellular proliferation22 revealed that UV irradiated anodized titanium induced significant increase in cellular proliferation (P=.009, at day 2, 5, and 7). Alkaline phosphatase which is associated with osteoblastic differentiation, bone formation, and matrix mineralization23, was composed in significantly higher level in UV irradiated anodized titanium disc (P=.016 at day 3, P=.009 at day 7 and 14). The Cellular attachment, proliferation, and differentiation were promoted by UV irradiation on anodized titanium disc.
The surface roughness did not show significant change after UV irradiation and the phase of oxide layers were not affected by UV irradiation. Both surface topography and phase component of anodized titanium did not show definite change after UV irradiation. UV irradiation induced change of chemical elemental composition of anodized titanium implant. Carbon decreased to about 1/3 level in mass concentration, and 2/5 level in atomic concentration after UV irradiation. The use of UV sterilizer as UV source might have induced this phenomenon. UV light is classified into UV-A, B, and C according to the wavelength, and UV-C (100 - 280 nm) is used as a light source of UV sterilizer. UV-C directly decomposes hydrocarbon and decrease of hydrocarbon seems to be one of the mechanism that increase the hydrophilicity of TiO2 after UV irradiation.24 The bioactivity of titanium degrades in proportion to time. 4-week-old titanium surface showed 50% less protein adsorption and osteoblastic migration/attachment than fresh surface, and the reduction of bioactivity was associated with the conversion of titanium discs from hydrophilicity to hydrophobicity.25 In other study, time-related degradation of bioactivity was associated with progressive accumulation of hydrocarbon on titanium surface.26 Although the exact mechanism how UV light enhances hydrophilicity of titanium is not clearly known, UV irradiation is very simple and easy way to enhance cellular response to titanium surface. This in vitro study also shows the positive effect of UV irradiation on anodized titanium and this effect can be achieved with a general UV sterilizer. Further in vivo study is needed to evaluate the initial and long term effect of UV irradiation on anodized titanium implant.
CONCLUSION
The hydrophilicity of anodized titanium discs increased after UV irradiation for 24 hours. Cells incubated on UV irradiated anodized titanium discs showed enhanced attachment. Cellular proliferation and differentiation also elevated on UV irradiated anodized titanium discs.
Footnotes
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0007781).
References
- 1.Brånemark PI, Hansson BO, Adell R, Breine U, Lindström J, Hallén O, Ohman A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1–132. [PubMed] [Google Scholar]
- 2.Albrektsson T, Wennerberg A. Oral implant surfaces: Part 1-review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004;17:536–543. [PubMed] [Google Scholar]
- 3.Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, Boyan BD. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005;74:49–58. doi: 10.1002/jbm.a.30320. [DOI] [PubMed] [Google Scholar]
- 4.Kieswetter K, Schwartz Z, Dean DD, Boyan BD. The role of implant surface characteristics in the healing of bone. Crit Rev Oral Biol Med. 1996;7:329–345. doi: 10.1177/10454411960070040301. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald DE, Deo N, Markovic B, Stranick M, Somasundaran P. Adsorption and dissolution behavior of human plasma fibronectin on thermally and chemically modified titanium dioxide particles. Biomaterials. 2002;23:1269–1279. doi: 10.1016/s0142-9612(01)00317-9. [DOI] [PubMed] [Google Scholar]
- 6.Wang R, Hashimoto K, Fujishima A, Chikuni M, Kojima E, Kitamura A, Shimohigoshi M, Watanabe T. Light-induced amphiphilic surfaces. Nature. 1997;388:431–432. [Google Scholar]
- 7.Shultz AN, Jang W, Hetherington WM, III, Baer DR, Wang LQ, Engelhard MH. Comparative second harmonic generation and X-ray photoelectron spectroscopy studies of the UV creation and O2 healing of Ti3+ defects on (110) rutile TiO2 surfaces. Surf Sci. 1995;339:114–124. [Google Scholar]
- 8.Hugenschmidt MB, Gamble L, Campbell CT. The interaction of H2O with a TiO2(110) surface. Surf Sci. 1994;302:329–340. [Google Scholar]
- 9.Henderson MA. An HREELS and TPD study of water on TiO2 (110) : the extent of molecular versus dissociative adsorption. Surf Sci. 1996;355:151–166. [Google Scholar]
- 10.Bullock EL, Patthey L, Steinemann SG. Clean and hydroxylated rutile TiO2(110) surfaces studied by X-ray photoelectron spectroscopy. Surf Sci. 1996;352-4:504–510. [Google Scholar]
- 11.Guillemot F, Porté MC, Labrugére C, Baquey CH. Ti4+ to Ti3+ conversion of TiO2 uppermost layer by low-temperature vacuum annealing: interest for titanium biomedical applications. J Colloid Interface Sci. 2002;255:75–78. doi: 10.1006/jcis.2002.8623. [DOI] [PubMed] [Google Scholar]
- 12.Kasemo B, Lausmaa J. Aspects of surface physics on titanium implants. Swed Dent J Suppl. 1985;28:19–36. [PubMed] [Google Scholar]
- 13.Zitter H, Plenk H., Jr The electrochemical behavior of metallic implant materials as an indicator of their biocompatibility. J Biomed Mater Res. 1987;21:881–896. doi: 10.1002/jbm.820210705. [DOI] [PubMed] [Google Scholar]
- 14.Solar RJ, Pollack SR, Korostoff E. In vitro corrosion testing of titanium surgical implant alloys: an approach to understanding titanium release from implants. J Biomed Mater Res. 1979;13:217–250. doi: 10.1002/jbm.820130206. [DOI] [PubMed] [Google Scholar]
- 15.Tengvall P, Lundström I. Physico-chemical considerations of titanium as a biomaterial. Clin Mater. 1992;9:115–134. doi: 10.1016/0267-6605(92)90056-y. [DOI] [PubMed] [Google Scholar]
- 16.Park KH, Heo SJ, Koak JY, Kim SK, Lee JB, Kim SH, Lim YJ. Osseointegration of anodized titanium implants under different current voltages: a rabbit study. J Oral Rehabil. 2007;34:517–527. doi: 10.1111/j.1365-2842.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee JE, Heo SJ, Koak JY, Kim SK, Han CH, Lee SJ. Healing response of cortical and cancellous bone around titanium implants. Int J Oral Maxillofac Implants. 2009;24:655–662. [PubMed] [Google Scholar]
- 18.Friberg B, Jemt T. Clinical experience of TiUnite implants: a 5-year cross-sectional, retrospective follow-up study. Clin Implant Dent Relat Res. 2010;12:e95–e103. doi: 10.1111/j.1708-8208.2009.00222.x. [DOI] [PubMed] [Google Scholar]
- 19.Li LH, Kong YM, Kim HW, Kim YW, Kim HE, Heo SJ, Koak JY. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials. 2004;25:2867–2875. doi: 10.1016/j.biomaterials.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 20.Kilpadi DV, Lemons JE. Surface energy characterization of unalloyed titanium implants. J Biomed Mater Res. 1994;28:1419–1425. doi: 10.1002/jbm.820281206. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson C, Nygren H, Ohlson K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials. 2004;25:4759–4766. doi: 10.1016/j.biomaterials.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Heo SJ, Koak JY, Kim SK, Lee SJ, Lee SH. Cellular responses on anodized titanium discs after laser irradiation. Lasers Surg Med. 2008;40:738–742. doi: 10.1002/lsm.20721. [DOI] [PubMed] [Google Scholar]
- 24.Aita H, Att W, Ueno T, Yamada M, Hori N, Iwasa F, Tsukimura N, Ogawa T. Ultraviolet light-mediated photofunctionalization of titanium to promote human mesenchymal stem cell migration, attachment, proliferation and differentiation. Acta Biomater. 2009;5:3247–3257. doi: 10.1016/j.actbio.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Hori N, Ueno T, Suzuki T, Yamada M, Att W, Okada S, Ohno A, Aita H, Kimoto K, Ogawa T. Ultraviolet light treatment for the restoration of age-related degradation of titanium bioactivity. Int J Oral Maxillofac Implants. 2010;25:49–62. [PubMed] [Google Scholar]
- 26.Att W, Hori N, Takeuchi M, Ouyang J, Yang Y, Anpo M, Ogawa T. Time-dependent degradation of titanium osteoconductivity: an implication of biological aging of implant materials. Biomaterials. 2009;30:5352–5363. doi: 10.1016/j.biomaterials.2009.06.040. [DOI] [PubMed] [Google Scholar]