Abstract
AIM
To evaluate the potential effects of therapeutic and supratherapeutic doses of linagliptin (BI 1356) on the QT/QTc interval in healthy subjects.
METHODS
The study was a randomized, double-blind, placebo-controlled, four-period crossover study using single oral doses of linagliptin (5 mg and 100 mg), moxifloxacin (400 mg) and placebo. Electrocardiogram (ECG) profiles using triplicates of 12-lead 10-s ECGs were digitally recorded pre-dose and after drug administration. The mean change from baseline (MCfB) of the individually heart rate corrected QT interval (QTcI) between 1 and 4 h postdrug administration was the primary end point. Blood samples to measure plasma concentrations of linagliptin and its main metabolite were also obtained.
RESULTS
Forty-four Caucasian subjects (26 male) entered the study and 43 subjects completed the study as planned in the protocol. Linagliptin was not associated with an increase in the baseline-adjusted mean QTcI, at any time point. The placebo-corrected MCfB of QTcI was −1.1 (90% CI −2.7, 0.5) ms and −2.5 (–4.1, –0.9) ms for linagliptin 5 mg and 100 mg, respectively, thus within the non-inferiority margin of 10 ms according to ICH E14. Linagliptin was well tolerated; the assessment of ECGs and other safety parameters gave no clinically relevant findings at either dose tested. Maximum plasma concentrations after administration of 100-mg linagliptin were ∼24-fold higher than those observed previously for chronic treatment with the therapeutic 5-mg dose. Assay sensitivity was confirmed by a placebo-corrected MCfB of QTcI with moxifloxacin of 6.9 (90% CI 5.4, 8.5) ms.
CONCLUSIONS
Therapeutic and significantly supratherapeutic exposure to linagliptin is not associated with QT interval prolongation.
Keywords: BI 1356, ECG, linagliptin, thorough QT study
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Linagliptin (BI 1356) is an oral, highly selective dipeptidyl peptidase-4 inhibitor which is under development for the treatment of type 2 diabetes mellitus and for which the pivotal phase III programme has recently been completed.
There have been no observed electrocardiogram changes in a linagliptin single rising dose study with up to 600 mg, and no preclinical signals for QT liability.
WHAT THIS STUDY ADDS
This manuscript describes the findings of a thorough QT study for linagliptin conducted according to the ICH E14 guideline, with a therapeutic dose (5 mg) and a 20-fold therapeutic dose (100 mg).
Linagliptin does not cause clinically relevant changes of the corrected QT interval with a therapeutic dose and a 20-fold therapeutic dose.
The 20-fold therapeutic dose of linagliptin was safe and well tolerated.
Introduction
Linagliptin (BI 1356) is an oral, xanthine-based, potent and highly selective dipeptidyl peptidase-4 (DPP-4) inhibitor that is being developed for the treatment of type 2 diabetes mellitus (T2DM) [1]. Its pharmacokinetic and pharmacodynamic profile permits once-daily dosing without the need for dose titration [2, 3]. DPP-4 inhibitors act by inhibiting the degradation of glucagon-like peptide-1 (GLP-1) and glucose insulinotropic peptide (GIP) by the plasma DPP-4 enzyme [4–6]. The increased availability of these hormones stimulates glucose-dependent insulin release [7, 8].
We performed a ‘thorough QT study’ (TQTS) for linagliptin to investigate the effect of linagliptin on the QT interval based on the International Conference of Harmonization (ICH) E14 guidance [9]. QT prolongation has been associated with arrhythmias, the most characteristic of which is the potentially fatal polymorphic tachycardia also known as torsade de pointes [10].
The importance of establishing the cardiac safety of any antidiabetic drug is particularly emphasized because of the target population of T2DM patients having an increased risk for cardiovascular disease, therefore making these individuals more susceptible to the effects which any drug may have on heart function.
Clinical trials of single rising oral doses (SRD) and multiple rising oral doses (MRD) of linagliptin involving healthy subjects and patients with T2DM[1, 11] have indicated that linagliptin is well tolerated at single doses up to 600 mg and multiple doses up to 10 mg once daily, with no clinically relevant changes observed in any of the electrocardiogram (ECG) parameters [12]. In both studies, digital 10-s ECGs were obtained and centrally measured using a semi-manual approach, both pre-dose and multiple times postdose. The statistical analyses of ECGs in these studies suggested the absence of a QT-prolonging effect with linagliptin. Additional information was provided by the absence of electrocardiographic findings in preclinical investigations; these included the lack of interaction on human ether-a-go-go-related gene (hERG)-mediated potassium current, the lack of a prolonging effect on the myocardial action potential in the guinea pig papillary muscle and beagle dog telemetry data for both linagliptin and its main metabolite, CD 1790 (unpublished data). The once-daily 5-mg dose studied in the present study is supported by the finding that >80% inhibition of plasma DPP-4 activity is maintained over a 24-h interval after drug intake at this dose [11]. Furthermore, the once-daily 5-mg dose produced and maintained significant improvements in glycaemic control in both a 12-week phase II study [13] and recently completed 24-week pivotal phase III studies [14–17].
This randomized, double-blind, crossover TQTS was conducted to confirm the cardiac safety of therapeutic and supratherapeutic doses of linagliptin. A crossover study design was used to demonstrate that the influence of linagliptin on the QTc interval was comparable with placebo. The 100-mg supratherapeutic dose of linagliptin used in this study is 20-fold that of the proposed therapeutic dose of 5 mg. For a TQTS, the use of supratherapeutic doses of the new agent up to 10-fold that of the proposed therapeutic dose has been suggested [18]. However, only a few QT safety studies have tested to this magnitude. An eightfold therapeutic dose of the DPP-4 inhibitor sitagliptin[19] was tested in a TQTS, and studies of the effects of antihistamines have used doses close to the suggested therapeutic range [20]. A single-dose design was appropriate as linagliptin shows low potential for accumulation and exposure does not rise substantially under steady-state conditions after multiple dosing [11]. The fluoroquinolone antibiotic moxifloxacin, which is known to prolong the QTc interval in a reproducible way, was used as a positive control to confirm the assay sensitivity of this study [21].
Methods
Study design
This was a single-centre, randomized, double-blind, placebo-controlled, four-period crossover study where subjects were exposed to single doses of each of the following four treatments on four different study days: linagliptin 5 mg, linagliptin 100 mg, moxifloxacin (Avelox®;Bayer Vital, Leverkusen, Germany) 400 mg and placebo. All treatments were administered after a light standard breakfast with 240-ml water and subjects were monitored for 24 h postdose.
The study involved double-blind administration of linagliptin and placebo, but the administration of moxifloxacin was open-label. The four treatments were formed to 12 sequences based on three orthogonal Latin Squares (Figure 1C). This design is variance-balanced and it ensures that each ordered combination of two treatments is given at regular intervals. The design has enough degrees of freedom to assess direct-by-carry-over effects, as well as to adjust treatment effects for potential carry-over [22, 23]. Moreover, this design ensures double-blind conditions of placebo and linagliptin treatments despite the open-label moxifloxacin. This is because the administration of moxifloxacin in each period is combined with all treatments in each of the periods. Other than the moxifloxacin administration and the absence of pharmacokinetic blood samplings (which were carried out for the linagliptin arms only), the experimental conditions in all study arms were kept the same.
Figure 1.
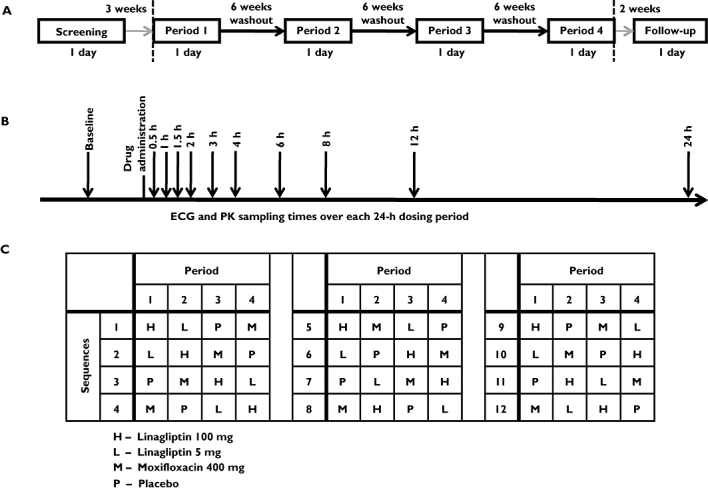
Elements of the study design: (A) Schedule of the visits, (B) timing of the electrocardiogram (ECG) and pharmacokinetic (PK) measurements and (C) randomization sequences, based on three orthogonal Latin squares
Each study day lasted for 24 h, during which ECG and pharmacokinetic assessments were made (Figure 1B).
Because of the long terminal half-life of linagliptin of 131 h [11], treatment periods were separated by washout periods of 6 weeks to ensure that no pharmacokinetic carry-over was present in any subject (Figure 1A). An end-of-study examination was performed within 14 days after the last drug administration.
Subjects
Forty-four healthy male and female subjects were recruited from the volunteers' pool of the Human Pharmacology Centre, Boehringer Ingelheim Pharma GmbH & Co., Biberach, Germany. Screening examinations were performed to confirm the health status and eligibility of the subjects.
Every subject provided written informed consent prior to participation in the study. The protocol was approved by the local ethics committee, Ethik-Kommission der Landesärztekammer Baden Württemberg, and by the German Competent Authority. The study was conducted in compliance with both the Good Clinical Practice and the ethical standards established by the Declaration of Helsinki (2004 version), as well as in accordance with applicable regulatory requirements. This study is included in the European Clinical Trials Database (EudraCT) as record number 2007-004220-21.
Electrocardiogram assessment
On each treatment day, three consecutive triple 12-lead 10-s ECGs were recorded 1 h prior to administration of study drug and 10 triple ECGs were recorded to assess the ECG profile at predefined time points after drug administration (0.5, 1, 1.5, 2, 3, 4, 6, 8, 12 and 24 h). Triplicate ECGs were recorded 30–120 s apart to account for intrinsic variability of the ECG intervals. All ECGs were recorded digitally with 500 Hz, after the subject had been resting in the supine position for at least 10 min, using a Corina Cardiosoft Electrocardiograph and the MUSE CV Cardiology System (General Electric Medical Systems, Freiburg, Germany).
The digital ECG recordings were transmitted electronically to a specified ECG core laboratory for semi-manual measurement of the ECG intervals (RR, PR, QRS and QT). The over-read was conducted by a single specialized technician for all ECGs in the study. As a result, the quality of ECG recordings was high and the QT interval was not measurable in only one of 6825 ECGs. Measurement of intervals for each session was performed on four consecutive ECG complexes from lead II. All 12 waveform measurements of the triple ECGs were averaged to obtain the ECG parameters at each time point.
One ECG of each triplicate was randomly selected for a cardiology assessment by a board-certified cardiologist. Additional ECG traces could have been performed for safety reasons at any time point, based on the judgement of the investigator. All ECGs complied with the Food and Drug Administration (FDA) guidance documents for annotated digital ECGs [9].
The baselines for the ECG end points were derived from three pre-dose triple ECGs in each study period. This has been shown to be a statistically efficient design, as the overall number of ECGs is lower compared to the use of a full baseline day prior to the treatment days [24, 25].
The study participants received a light breakfast after the pre-dose ECGs, a light lunch 4 h postdose, a snack at 6 h postdose, a dinner at 10 h postdose and a snack at 12 h postdose. All meals were taken after the samplings for ECG and PK were taken.
Pharmacokinetic evaluation
Blood samples for pharmacokinetic measurements were collected at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12 and 24 h after linagliptin administration, at the same time as the ECG measurements, on each of the treatment days. Sample and data analyses were conducted as previously reported [1]. The calibration curves of undiluted plasma samples were linear over the range of concentrations from 0.100 to 100 nmol l−1 for linagliptin and from 0.0500 to 50.0 nmol l−1 for CD 1750 using a plasma volume of 150 µl. In-study assay validation at nominal concentrations of 0.25, 1.0, 5 and 80.0 nmol l−1 yielded an assay inaccuracy and imprecision for linagliptin of −5.5 to 2.4% and 6.0 to 8.3% respectively. For CD 1790, nominal concentrations of 0.125, 0.5, 2.5 and 40.0 nmol l−1 resulted in assay inaccuracy and imprecision of 0.0 to −8.0 and 5.3 to 9.0% respectively.
Non-compartmental pharmacokinetic analyses were used to derive pharmacokinetic parameters, including observed maximum plasma concentration (Cmax), time to observed Cmax (tmax) and area under the plasma concentration–time curve over a 24-h dosing period [AUC(0,24 h)], for linagliptin and its main metabolite, CD 1790. As the pharmacokinetics of both analytes had been characterized previously, pharmacokinetic samples were obtained only up to 24 h postdrug administration. Thereby, it was ensured that an exposure–response analysis could be performed, while pharmacokinetic parameters were only to be derived for this time frame [e.g. AUC(0,24 h) instead of AUC(0,∞)]. Geometric mean (gMean) and geometric coefficient of variation (gCV) values were determined for the pharmacokinetic parameters.
Safety evaluation
Adverse events were recorded throughout the study. Vital signs (pulse rate, systolic and diastolic blood pressure) were recorded after the subject had been at rest in a supine position for at least 10 min at screening and at the end-of-study evaluation. Clinical laboratory parameter assessments and pregnancy testing of female subjects were conducted after a fasting period of at least 10 h at screening, pre-dose and at end-of-study evaluations. Tolerability was assessed by the investigator and was based on adverse event reporting and laboratory evaluations on completion of the study assessment. Tolerability was assessed using the categories ‘good’, ‘satisfactory’, ‘not satisfactory’ or ‘bad’.
Statistical analysis
Primary and secondary end points
The primary end point was derived as the mean of the baseline-adjusted subject-specific heart rate-corrected QT interval (QTcI) over 1 to 4 h postdose, where baseline values were derived as the mean of three ECGs obtained pre-dose in the same study period. In this 3-h post-dose time window, the plasma concentrations of both linagliptin and moxifloxacin were expected to be at least 75% of their peak plasma concentrations, ensuring the most relevant levels of exposure to study drug.
Secondary end points included the change from mean baseline of the QTcI at any point within the complete profile time interval of 30 min to 24 h after dosing. In addition, the occurrence/non-occurrence of at least one notable change in QTcI, QTcN, QTcF, QTcB (for explanations of these symbols, please see below) or the uncorrected QT interval in any non-baseline value was recorded. Notable changes were determined using the following parameters in accordance to ICH E14: new onset of QTcI greater than 500, 480 or 450 ms postbaseline, or change from baseline of QTc greater than 60 or 30 ms based on the mean values of each triplicate ECGs.
Statistical models
The inferential analysis of the ECG intervals consisted of four parts. First, the individual and the population heart rate corrections of the QT interval were determined using a multi-level model. The analysis of the primary end point (mean QTcI change from baseline between 1 and 4 h posttreatment) was carried out using an analysis of covariance (ancova) taking into account the effects of baseline, treatment, period and sequence. The analysis of the QTcI change from baseline over time was performed using a repeated measurements approach. Finally, the linear exposure–response analysis was based on an ancova, with covariate pharmacokinetic concentration. All four analyses are specific examples of mixed-effects models.
The heart rate correction was performed on log-transformed QT- and RR-interval data using a mixed model accounting for subject and subject by slope interaction as fixed effects, as well as period and time points as random effects with compound symmetry covariance structure [26, 27]. Similarly, the study population heart rate-corrected QT interval (QTcN) was derived using a multi-level model accounting for subject as intercept. The heart rate correction was derived on all pre-dose data, and the validity of the correction was checked by a correlation analysis of on-treatment QTc and RR interval data. QTcI was chosen as the primary parameter instead of a fixed heart rate correction, such as the Fridericia correction (QTcF), as the latter ones can lead to biased estimations of QTc effects if the QT–RR relationship of the study subjects deviates substantially from that assumed by the fixed correction approach [27, 28].
The primary objective of this study was addressed by testing the following null hypothesis: the difference between treatment with linagliptin (5 mg and 100 mg) and placebo in the mean change from baseline for the QTcI interval was greater than or equal to 10 ms using one-sided testing at the 5% significance level, based on two-sided 90% confidence intervals. The conclusion that linagliptin (5 mg and 100 mg) was not inferior to placebo was to be based on the rejection of the null hypothesis for each of the doses. The non-inferiority margin was chosen at 10 ms in accordance with the ICH E14 guideline [9].
The statistical model for the secondary end points ‘QTcI at any point in time’ was a repeated-measurements model, with similar effects as the primary analysis but also accounting for the covariance between the time points (unstructured covariance matrix). From this analysis, the largest time-matched difference between drug and placebo was also determined as a requirement of the ICH E14 guideline.
The relationship between plasma linagliptin (5 mg and 100 mg) and placebo-adjusted QTcI change from baseline was investigated in an exploratory way using a linear mixed-model approach with subjects as random intercept effect to estimate the QTcI change from baseline and its 90% CI at the gMean of the Cmax of both doses of linagliptin.
All analyses were also performed for the uncorrected QT interval, the RR interval and the heart rate, as well as other quantitative QTc intervals: the population correction QTcN, the Fridericia correction (QTcF) and the Bazett correction (QTcB). Sensitivity of the study to detect moxifloxacin-induced changes in QTcI was assessed using the same ancova models.
Sample size
Based on the absence of QT effects in pre-clinical investigations and in the single and multiple dose safety trials, an expected difference of about 1 ms between linagliptin and placebo was used for the sample size calculation. The standard deviation for the primary end point was expected with a value of 9.5 ms based on previous trials at Boehringer Ingelheim in a healthy subject population. To achieve a power of 90% of concluding non-inferiority of the primary end point using the regulatory margin of 10 ms, data for 40 evaluable subjects needed to be obtained. Therefore forty-four subjects were entered into the study to account for potential dropouts. Furthermore, a sample size of 40 evaluable subjects was considered sufficient to permit detection of a mean QTcI change from baseline of moxifloxacin, which is significantly larger than 0 ms with a power of 90% using a t-test with a 0.05 one-sided significance level, based on a placebo-adjusted effect size for moxifloxacin of greater than or equal to 9 ms.
Results
Subject demographics and disposition
Forty-four healthy subjects (26 male, 59.1%) with a mean (range) age of 36.4 (22–48) years, mean weight of 72.2 (51–99) kg and mean body mass index (BMI) of 23.6 (19.0–28.9) kg m–2 were entered into this study. Forty-three subjects completed the study, while one female subject was withdrawn from the study because of a serious adverse event (detection of breast cancer) which was not considered to be related to the study drug. This subject had completed three of the four study periods: linagliptin 100 mg, placebo and moxifloxacin 400 mg.
Individually heart rate-corrected QT interval (QTcI)
The primary end point, the mean QTcI change from baseline between 1 and 4 h postdose of linagliptin was −1.1 ms (90% CI −2.7, 0.5 ms) for the 5-mg dose and −2.5 ms (90% CI −4.1, −0.9 ms) for the 100-mg dose, when compared with placebo. The 90% confidence intervals of this end point were well below the pre-defined standard non-inferiority margin of 10 ms (Table 1).
Table 1.
Adjusted means and confidence intervals for the mean QTcI change from baseline between 1 and 4 h postdose
| Adjusted mean ΔQTcI (ms) | ΔQTcI difference from placebo (ms) | |||
|---|---|---|---|---|
| Treatment | n | Mean | Mean | 90% CI (lower, upper) |
| Placebo | 44 | –5.6 | ||
| Linagliptin 5 mg | 43 | –6.7 | –1.1 | –2.7, 0.5 |
| Linagliptin 100 mg | 44 | –8.1 | –2.5 | –4.1, −0.9 |
| Moxifloxacin 400 mg | 44 | 1.3 | 6.9 | 5.4, 8.5 |
The mean changes in the QTcI at any point in time compared with placebo ranged from −2.0 to 0.2 ms for linagliptin 5 mg and −4.7 to −0.9 ms for linagliptin 100 mg. The ICH E14 guideline focuses on evaluating whether QT/QTc interval prolongation occurs [9], for linagliptin 5 mg, the largest placebo-adjusted change from baseline towards QTcI prolongation was observed at 1 h postdose with 0.2 ms (90% CI −2.0, 2.4 ms), and for linagliptin 100 mg at 6 h postdose with −0.9 ms (90% CI −3.8, –2.0 ms; Figure 2, Table 2). All values were below the pre-defined standard non-inferiority margin of 10 ms. A subgroup analysis with respect to gender showed very similar results and no differences between the genders.
Figure 2.
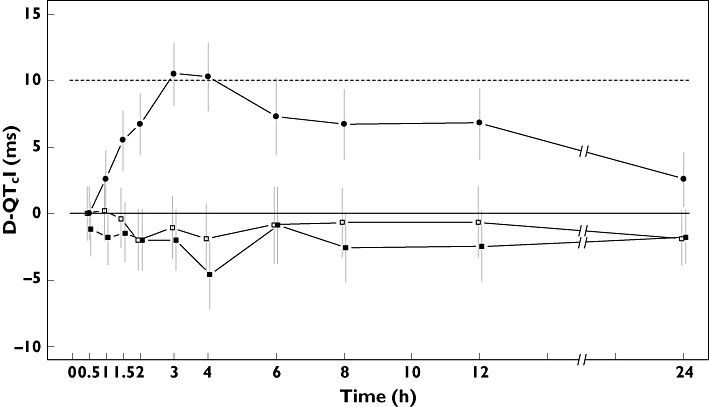
Change from baseline of placebo-adjusted QTcI after dosing with linagliptin 5 mg (□) or 100 mg ( ) and moxifloxacin 400 mg (•) (adjusted mean change and the 90% confidence interval are shown over time)
) and moxifloxacin 400 mg (•) (adjusted mean change and the 90% confidence interval are shown over time)
Table 2.
Comparison of the outcome (mean, 90% CI) of the placebo-corrected QTc (as well as uncorrected QT interval and heart rate) change from baseline for linagliptin 5 mg and 100 mg, and moxifloxacin 400 mg over time
| Linagliptin 5 mg (n = 43) | |||||
|---|---|---|---|---|---|
| Time (h) | QTcI (ms) | QTcN (ms) | QTcF (ms) | QT (ms) | HR (beats min−1) |
| 0.5 | 0.0 (–2.0, 2.0) | 0.3 (–1.5, 2.1) | 0.8 (–1.2, 2.8) | –0.3 (–2.8, 2.2) | 0.6 (–0.7, 1.8) |
| 1 | 0.2 (–2.0, 2.4) | 0.1 (–1.9, 2.2) | 1.3 (–0.8, 3.4) | –1.4 (–4.3, 1.5) | 1.3 (0.0, 2.6) |
| 1.5 | –0.4 (–2.7, 1.9) | –0.1 (–2.3, 2.1) | –0.1 (–2.2, 1.9) | 0.1 (–3.2, 3.4) | 0.0 (–1.3, 1.4) |
| 2 | –2.0 (–4.3, 0.3) | –2.4 (–4.5, −0.2) | –1.6 (–3.8, 0.6) | –3.3 (–6.4, −0.2) | 0.9 (–0.4, 2.1) |
| 3 | –1.0 (–3.4, 1.3) | –1.2 (–3.4, 1.1) | –0.9 (–3.0, 1.2) | –1.4 (–4.6, 1.8) | 0.4 (–0.7, 1.4) |
| 4 | –1.8 (–4.4, 0.7) | –1.8 (–4.2, 0.7) | –1.9 (–4.1, 0.3) | –1.4 (–5.0, 2.1) | –0.3 (–1.4, 0.9) |
| 6 | –0.9 (–3.8, 2.0) | –0.3 (–2.6, 2.1) | 0.9 (–1.2, 3.0) | –1.8 (–5.5, 1.9) | 1.2 (–0.4, 2.8) |
| 8 | –0.7 (–3.3, 1.9) | –0.6 (–2.9, 1.6) | 0.4 (–1.7, 2.5) | –2.0 (–5.4, 1.5) | 1.1 (–0.4, 2.7) |
| 12 | –0.6 (–3.3, 2.0) | –0.6 (–3.1, 1.8) | –0.4 (–2.6, 1.7) | –0.7 (–4.3, 2.9) | 0.1 (–1.3, 1.6) |
| 24 | –1.8 (–3.9, 0.2) | –1.7 (–3.8, 0.4) | –1.8 (–3.7, 0.2) | –1.5 (–4.6, 1.6) | 0.0 (–1.3, 1.2) |
| Linagliptin 100 mg (n = 44) | |||||
|---|---|---|---|---|---|
| Time (h) | QTcI (ms) | QTcN (ms) | QTcF (ms) | QT (ms) | HR (beats min−1) |
| 0.5 | –1.2 (–3.2, 0.8) | –0.9 (–2.7, 0.9) | 1.6 (–0.4, 3.6) | –4.2 (–6.7, −1.7) | 3.1 (1.9, 4.3) |
| 1 | –1.8 (–3.9, 0.4) | –1.4 (–3.4, 0.6) | 1.8 (–0.3, 3.8) | –5.7 (–8.6, −2.9) | 4.0 (2.7, 5.3) |
| 1.5 | –1.4 (–3.7, 0.8) | –1.1 (–3.3, 1.2) | 1.0 (–1.1, 3.0) | –3.8 (–7.0, −0.5) | 2.7 (1.3, 4.1) |
| 2 | –2.0 (–4.3, 0.3) | –1.6 (–3.7, 0.6) | 1.1 (–1.1, 3.2) | –5.3 (–8.3, −2.2) | 3.2 (1.9, 4.5) |
| 3 | –2.0 (–4.3, 0.3) | –1.5 (–3.7, 0.8) | 0.8 (–1.3, 2.9) | –4.6 (–7.8, −1.4) | 2.6 (1.6, 3.7) |
| 4 | –4.7 (–7.2, −2.1) | –4.2 (–6.7, −1.8) | –2.8 (–5.0, −0.6) | –6.2 (–9.7, −2.7) | 1.7 (0.5, 2.8) |
| 6 | –0.9 (–3.8, 2.0) | 0.0 (–2.4, 2.3) | 1.4 (–0.6, 3.5) | –2.0 (–5.7, 1.7) | 1.9 (0.3, 3.5) |
| 8 | –2.6 (–5.2, 0.0) | –1.7 (–3.9, 0.5) | 0.8 (–1.2, 2.9) | –5.2 (–8.7, −1.7) | 3.2 (1.7, 4.8) |
| 12 | –2.5 (–5.1, 0.1) | –1.6 (–4.0, 0.8) | –0.9 (–3.1, 1.2) | –2.4 (–5.9, 1.2) | 1.0 (–0.4, 2.4) |
| 24 | –1.8 (–3.8, 0.3) | –1.4 (–3.5, 0.7) | –0.2 (–2.1, 1.8) | –3.0 (–6.1, 0.1) | 1.4 (0.2, 2.7) |
| Moxifloxacin 400 mg (n = 44) | |||||
|---|---|---|---|---|---|
| Time (h) | QTcI (ms) | QTcN (ms) | QTcF (ms) | QT (ms) | HR (beats min−1) |
| 0.5 | 0.0 (–2.0, 2.0) | 0.1 (–1.7, 1.9) | 0.6 (–1.3, 2.6) | –0.4 (–2.9, 2.1) | 0.5 (–0.7, 1.7) |
| 1 | 2.6 (0.4, 4.7) | 2.1 (0.1, 4.1) | 3.0 (0.9, 5.1) | 1.0 (–1.8, 3.9) | 1.0 (–0.3, 2.3) |
| 1.5 | 5.5 (3.2, 7.7) | 5.4 (3.1, 7.6) | 5.9 (3.9, 7.9) | 4.8 (1.5, 8.0) | 0.5 (–0.9, 1.9) |
| 2 | 6.7 (4.4, 9.0) | 6.3 (4.2, 8.5) | 8.1 (6.0, 10.3) | 3.9 (0.8, 7.0) | 1.8 (0.6, 3.1) |
| 3 | 10.4 (8.1, 12.7) | 10.5 (8.3, 12.8) | 11.5 (9.4, 13.6) | 9.4 (6.2, 12.6) | 0.9 (–0.1, 2.0) |
| 4 | 10.3 (7.7, 12.8) | 10.1 (7.6, 12.5) | 11.0 (8.8, 13.2) | 8.9 (5.4, 12.5) | 1.0 (–0.2, 2.1) |
| 6 | 7.3 (4.4, 10.2) | 7.8 (5.4, 10.2) | 8.3 (6.2, 10.4) | 7.2 (3.6, 10.9) | 0.4 (–1.2, 2.0) |
| 8 | 6.7 (4.1, 9.3) | 7.0 (4.8, 9.3) | 8.8 (6.7, 10.8) | 4.8 (1.3, 8.2) | 1.8 (0.3, 3.4) |
| 12 | 6.7 (4.1, 9.4) | 7.0 (4.6, 9.5) | 7.6 (5.5, 9.7) | 6.5 (2.9, 10.1) | 0.4 (–1.0, 1.8) |
| 24 | 2.5 (0.5, 4.6) | 2.2 (0.1, 4.3) | 2.4 (0.5, 4.3) | 2.1 (–0.9, 5.2) | 0.0 (–1.3, 1.2) |
Mean QTcI change from baseline between 1 and 4 h postdose of the positive control moxifloxacin 400 mg was significantly prolonged compared with placebo. The placebo-adjusted difference was 6.9 ms (P = 0.02), with a lower 90% CI limit of 5.4 ms (Table 1). The largest increase was observed at 3 h, with a placebo-adjusted difference of 10.4 ms (lower 90% CI limit = 8.1 ms; Figure 2, Table 2). These results confirm the assay sensitivity of the study, i.e. the ability to detect existing QT prolongation.
Other ECG parameters of interest
In addition to the primary parameter QTcI, the same repeated-measurements analysis was also carried out for the other QTc and heart rate end points. The results of other heart rate corrections (QTcN, QTcF and QTcB) were similar to those of QTcI, although the mean of individual slopes of the logarithmic QT–RR relationship was 0.20, in agreement with the population slope, i.e. much smaller than the 0.333 of the Fridericia correction. This finding is in agreement with previous studies carried out at Boehringer Ingelheim [26].
No new onset of QTcI greater than 500, 480 or 450 ms postbaseline and no changes of more than 30 ms with respect to baseline were observed in the QTcI, QTcN or QTcF intervals for linagliptin 5 mg, linagliptin 100 mg or placebo. After moxifloxacin 400-mg administration, one subject exceeded the QTcF change from baseline threshold of 30 ms, while another subject exceeded the 450-ms threshold for the QTcI interval.
There was a small increase of heart rate following the 100-mg dose of linagliptin. The maximum increase was seen at 1 h postdose with a value of 4 beats min−1 compared with placebo. Notably, the heart rate was already increased in all dose groups until 1.5 h postdose, e.g. the mean heart rate increase following placebo compared with the pre-dose baseline was between 4.8 and 5.1 beats min−1 in this time frame, and 5.3–5.9 beats min−1 following moxifloxacin. There was, however, no increase of the QTcI in this time frame, because the decrease of the QT interval compensated for this increase in the heart rate (Table 2).
The other ECG intervals (PR and QRS) remained virtually unchanged. The descriptive mean change from baseline of the PR intervals did not exceed 1.5 ms for both doses of linagliptin and placebo at any point in time, while the mean change from baseline of the QRS intervals did not exceed 0.5 ms. Hence, this study also showed that there was no increased risk of AV block with linagliptin administration.
Pharmacokinetic results
Pharmacokinetics samples were obtained in parallel with the ECG measurements. Because of the long terminal half-life of linagliptin, several subjects demonstrated small pre-dose concentrations despite a washout phase between periods of 6 weeks. As all pre-dose concentrations of linagliptin were below 5% of the Cmax after administration of 5 mg in each subject, this pharmacokinetic carry-over was considered to have no relevant pharmacodynamic effect in the respective treatment period.
The pharmacokinetic findings are summarized in Table 3. Maximum observed plasma concentration increased 38-fold, presenting a non-proportional increase which could be because of the non-linear pharmacokinetic properties that have previously been observed with linagliptin [1]. This Cmax result occurred with a 20-fold increase in dose of linagliptin (5 mg and 100 mg) and total exposure over 24 h increased approximately 17-fold. After administration of linagliptin 5 mg, the primary metabolite CD 1790 accounted for 7% of parent exposure in plasma. This value increased to 28% after administration of linagliptin 100 mg. Plasma concentrations of linagliptin generally declined in a biphasic manner after Cmax (Figure 3), as did those of CD 1790.
Table 3.
Linagliptin pharmacokinetics after single doses of linagliptin 5 mg and 100 mg
| Non-compartmental parameters of linagliptin | Non-compartmental parameters of CD 1750 | |||||||
|---|---|---|---|---|---|---|---|---|
| Linagliptin 5 mg (n = 43) | Linagliptin 100 mg (n = 44) | Linagliptin 5 mg (n = 43) | Linagliptin 100 mg (n = 44) | |||||
| Parameter | gMean | gCV (%) | gMean | gCV (%) | gMean | gCV (%) | gMean | gCV (%) |
| AUC(0,24 h) (nmol l−1 h) | 101 | 25.1 | 1760 | 59.2 | 7.0 | 56.2 | 484 | 65.9 |
| Cmax (nmol l−1) | 7.05 | 28.5 | 267 | 66.6 | 1.1 | 59.7 | 59.7 | 57.9 |
| tmax (h)* | 2.00 | 0.5–3.0 | 1.52 | 0.5–6.0 | 3.0 | 1.0–4.0 | 3.0 | 1.0–6.0 |
For tmax, the median and range (min–max) are given. AUC(0, 24 h), Area under the plasma concentration–time curve from 0 to 24 h; Cmax, maximum observed plasma concentration; tmax, time of maximum observed concentration.
Figure 3.
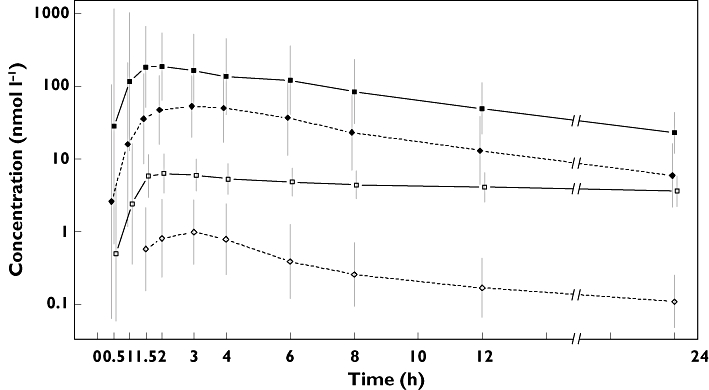
Drug plasma concentration–time profile of linagliptin and its metabolite CD 1790 after single oral administration of linagliptin 5 mg and 100 mg to healthy subjects (gMean ± gCV are shown on semi-log scale). Following 100-mg linagliptin ( ); CD 1790 following 100-mg linagliptin (
); CD 1790 following 100-mg linagliptin ( ); following 5-mg linagliptin (
); following 5-mg linagliptin ( ); CD 1790 following 5-mg linagliptin (
); CD 1790 following 5-mg linagliptin ( )
)
Pharmacokinetic–pharmacodynamic evaluation
Time points with the highest individual QTcI change from baseline did not correspond to time points close to either the linagliptin or CD 1790 maximum plasma concentrations. The pre-defined exposure–response analysis between linagliptin plasma concentrations (5 mg and 100 mg) and placebo-adjusted QTcI changes from baseline resulted in a slope of –0.0076 ms (nmol l−1)−1 (with 90% CI –0.016, 0.0005).
The estimated intercept at concentration 0 was −1.6 ms (small effects on heart rate and QT interval may occur in subjects receiving a blinded placebo under study conditions). The estimated placebo-adjusted QTcI change from baseline at maximum concentrations of linagliptin was −1.6 ms for the 5-mg dose and −3.6 ms for the 100-mg dose (Table 4), which agrees with the primary and secondary analyses.
Table 4.
Confidence intervals for the QTcI and HR change effects as predicted by exposure–response relationship of linagliptin, based on the placebo-corrected QTcI/HR change from baseline vs. concentration of linagliptin at gMean of Cmax
| Exposure – response analysis for QTcI | Exposure – response analysis for Heart Rate | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | gMean of Cmax (nmol l−1) | PK-QTcI intercept (ms) | PK-QTcI slope [ms/(nmol l−1)] | Predicted value of QTcI at Cmax (90% CI; ms) | PK-HR intercept (beats min−1) | PK-HR slope [beats min−1/(nmol l−1)] | Predicted value of HR at Cmax (90% CI; beats min−1) |
| Linagliptin 5 mg | 7 | –1.6 | –0.008 | –1.6 (–2.9, −0.4) | 0.8 | 0.0109 | 0.9 (0.0, 1.7) |
| Linagliptin 100 mg | 267 | –3.6 (–6.0, −1.2) | 3.7 (2.5, 4.9) | ||||
An exposure–response analysis was also carried out for the heart rate. The outcome of this analysis (Table 4) was similar to the E14-type analysis (Table 2). For the Cmax of the linagliptin 5-mg therapeutic dose, the change was estimated with a value of 0.9 beats min−1 (90% CI 0.0, 1.7 beats min−1), while for the Cmax of the 100-mg supratherapeutic dose, this change was 3.7 beats min−1 (90% CI 2.5, 4.9 beats min−1) compared with placebo. As the change was only affecting the supratherapeutic dose, this was not considered clinically relevant for therapeutic exposure of linagliptin.
A similar investigation for the metabolite CD 1790 indicated that there was no association between systemic exposure of CD 1790 and placebo-adjusted QTcI changes from baseline.
Safety and tolerability
Linagliptin 5-mg and 100-mg doses were well tolerated when administered as single doses. The drug-related adverse event most commonly reported was headache, which was observed in all treatment groups [five subjects on linagliptin 100 mg (11.4%), three subjects on linagliptin 5 mg (7.0%), two subjects on moxifloxacin (4.5%), two subjects on placebo (4.5%)]. One female subject was withdrawn from the study after the diagnosis of breast cancer. The subject had completed three of the four study periods (linagliptin 100 mg, placebo and moxifloxacin 400 mg) prior to diagnosis. Breast cancer was reported as a serious adverse event and was considered unrelated to study medication by the investigator.
Other observed adverse events were fatigue (one after 100-mg linagliptin and one after placebo), hypersensitivity (one after 100-mg linagliptin), epistaxis (one after 100-mg linagliptin), sciatica (one after 5-mg linagliptin) and haematoma (one after 5-mg linagliptin). All adverse events reported during the study, other than the occurrence of breast cancer, were rated as either mild or moderate in intensity.
There were no clinically significant laboratory abnormalities reported and no clinically relevant abnormalities observed in the ECGs of any of the subjects. Vital signs were normal throughout the study. Tolerability was rated as ‘good’ for all subjects and treatments by the investigator.
Discussion
This TQTS was performed to investigate the effect of linagliptin on the QTc interval, based on the ICH E14 guidance. During the development of linagliptin, no relevant ECG-related preclinical or clinical findings have previously been observed. Previous in vitro and animal studies with linagliptin have demonstrated the absence of a relevant interaction between linagliptin and the IKr channel, and no effects of linagliptin or its main metabolite CD 1790 (neither prolongation nor shortening) on the QT interval duration have been observed (unpublished data, Guth B, unpublished data, Van Ryn J). Furthermore, a detailed analysis of ECG recordings from previous clinical studies provided no suggestion that linagliptin might have an effect on ECG parameters, particularly with regard to the QT interval [1, 11].
In this TQTS, conducted in healthy subjects, neither the proposed therapeutic dose nor the significantly supratherapeutic dose of linagliptin showed any evidence of QT prolongation or other clinically relevant changes of the ECG. Taking these observations into account, it appears that linagliptin has little or no potential to precipitate arrhythmias associated with a prolongation of the QT interval.
For both 5-mg and 100-mg doses of linagliptin, there was no increase in QTcI compared with placebo. The results for linagliptin appear to be different from those of sitagliptin, another DPP-4 inhibitor. Sitagliptin caused a small but statistically significant increase in QTcF at an eightfold supratherapeutic dose (800 mg), with an upper limit of the one-sided 95% CI of 10.6 ms, exceeding the standard non-inferiority margin of 10 ms [19]. In contrast, saxagliptin and its active metabolite have been reported to have no dose- or concentration-dependent effect on QTcF or QTcI at an eightfold supratherapeutic dose (40 mg) [29]. Vildagliptin also has no effect on QTcI but an increased incidence of first-degree AV block has been reported, although an association between vildagliptin and first-degree AV block has yet to be confirmed or excluded [30].
At the peak exposure of the therapeutic 5-mg linagliptin dose, no statistically significant change of the QTcI interval was observed. For the 100-mg dose of linagliptin, the QTcI interval was shortened by up to 4.7 ms (90% CI −7.2, 2.1 ms) compared with placebo at a median time of 4 h after administration, which was statistically significant. Because of the small magnitude of the effect, which occurred only at the dose 20-fold higher than the therapeutic dose, this result was not considered to be clinically significant. Although there is some scientific discussion on the clinical interpretation of QT shortening, its impact on clinical outcomes is generally deemed low and intensive investigations of QTc-shortening drugs do not currently seem to be warranted for drug approval [31].
The shortening might be attributed by the increase of heart rate of up to 4.0 beats min−1 (90% CI 2.7, 5.3 beats min−1) compared with placebo, which was compensated by a decrease of the QT interval. Generally, the heart rates seen on treatment were larger than those at baseline, which could be explained by the circadian rhythm. Intake of food might contribute to this effect, although the meals given during the treatment periods were light. It was not deemed to be appropriate to maintain fasting conditions during the whole morning of the study day, as starving also might lead to substantial changes of ECG parameters.
Mean maximum linagliptin plasma concentrations obtained in the present study after administration of linagliptin 100 mg were approximately 38-fold higher compared with the therapeutic single dose of 5 mg administered in this study [Cmax 267 nmol l−1, AUC(0,24 h) 1760 nmol l−1 h; Table 3], and approximately 24-fold higher compared to steady-state exposure of 5 mg as determined previously [11]. This non-proportional increase in systemic exposure was attributed to the non-linear pharmacokinetic properties of linagliptin and was known from previous studies [1]. After administration of linagliptin 100 mg, the range for Cmax values was 266–1280 nmol l−1 and the range for AUC(0,24 h) values was 466–4970 nmol l−1 h. In contrast, the highest individual steady-state Cmax and AUC(0,24 h) reported so far after administration of the therapeutic linagliptin 5-mg dose did not exceed 80 nmol l−1 and 400 nmol l−1 h respectively. Regarding the main metabolite CD 1790, the highest individual steady-state Cmax and AUC(0,24 h) of CD 1790 observed so far in clinical studies after administration of 5-mg linagliptin have remained below 7 nmol l−1 and 50 nmol l−1 h respectively. In comparison, this present study resulted in mean (range) CD 1790 Cmax and AUC(0,24 h) values of 59.7 nmol l−1 (19.2–166 nmol l−1) and 484 nmol l−1 h (107–1410 nmol l−1 h), respectively, after administration of 100-mg linagliptin. Therefore, the exposure to linagliptin, and to its main metabolite CD 1790, achieved in this TQTS following supratherapeutic dosing of linagliptin, covered and significantly exceeded steady-state exposure following therapeutic linagliptin dosing. These data therefore provide reassurance for the therapeutic use of linagliptin in the clinical setting.
The study was performed in a crossover fashion. Because of the long terminal half-life of linagliptin, a 6-week washout period was used between dosing periods to ensure that no relevant pharmacokinetic carry-over was seen and the analysis was designed to take account of carry-over should this be observed. The disadvantage of the study duration of 6 months was considered to be outweighed by the advantages of intraindividual comparisons that would be possible using this study design, and the minimal pharmacokinetic carry-over observed, combined with the low dropout rate, confirmed the feasibility of this approach.
The baseline consisted of three triple ECGs in each treatment period to provide a robust evaluation of the change from baseline. This approach was later described as a powerful method to collect baselines in crossover studies [24] because the individual circadian rhythm can be accounted for with data from the placebo period.
The heart rate correction was based on baseline data only. However, it was confirmed that the on-treatment relationship of QT and RR intervals was similar to the off-treatment relationship. The slope of the QTcN (as determined by a multi-level model [26]) was equal to the mean of the slopes of the QTcI (with a value of 0.20, smaller than Fridericia), as indicated by the theory [9, 27, 28]. The reporting of previous disagreements between both approaches [32] may have been originated by imperfect application of regression analyses that did not account for the multi-level structure of the QT/RR data in clinical QT trials. Notably, the standard errors in the QTcN analyses were smaller at almost all time points than those of QTcI. The same was true for the primary end point in this trial, the mean of the QTc between 1 and 4 h postdose, for which the standard error of QTcN was about 6% smaller than that of QTcI. This indicates a better statistical efficiency of the population correction for the future choice of a primary parameter, which already has been suggested by various authors [28, 33, 34].
Because of potential gender differences in QT effects [35, 36], the present study was performed in both genders. Mean changes of QTcI interval from baseline as well as other ECG intervals were similar for both genders.
The ICH E14 guidance recommends that all QT trials should include a positive control to confirm the sensitivity of the study to detect changes in QT [9]. Moxifloxacin 400 mg was considered to be an appropriate positive control for this study as it has previously demonstrated its ability to effect such changes with this dose in healthy subjects [21, 37], and the QT effects seen in this study confirmed the assay sensitivity.
Linagliptin was well tolerated at the proposed therapeutic dose (5 mg) and at the supratherapeutic dose (100 mg). This tolerability profile was expected based on the results from a previous trial in which doses of up to 600 mg were investigated [1]. In this randomized, double-blind, parallel, placebo-controlled within dose groups, SRD study, 25 subjects received linagliptin at doses of 100 mg or higher (100 mg n = 8, 200 mg n = 6, 400 mg n = 5 and 600 mg n = 6). Therefore, the inclusion of a 100-mg dose in this TQTS was based on the actual observed safety profile for higher dosages of linagliptin. The current study has also provided additional value by confirming the safety of linagliptin at a supratherapeutic dose level because of its application in a larger study population. The tolerability findings agreed with those from multiple dose studies of linagliptin given once daily for up to 12 weeks at doses of up to 10 mg [13].
In conclusion, this TQTS, designed and carried out in accordance with the guidance ICH E14, showed that linagliptin administered at therapeutic and 20-fold supratherapeutic doses did not prolong the QT interval duration. The present study supports the favourable safety and tolerability profile of linagliptin observed to date.
Acknowledgments
The authors would like to thank the volunteers and staff who participated in this study, particularly Gerhard Ries for excellent operational support. Also, the authors acknowledge the assistance of Beate Walter for carrying out sections of the statistical analysis. Medical writing and editorial support for the manuscript was provided by Patrick Foley of PHASE II International Ltd, with the financial support of Boehringer Ingelheim. Data from this study have been presented (as Poster P292) at the 12th European Congress of Endocrinology (ECE), 24–28 April 2010, Prague. All authors saw and approved the final version of the manuscript.
Competing Interests
The authors are all employees of Boehringer Ingelheim Pharma GmbH & Co. KG.
REFERENCES
- 1.Hüttner S, Graefe-Mody EU, Withopf B, Ring A, Dugi KA. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single oral doses of BI 1356, an inhibitor of dipeptidyl peptidase-4, in healthy male volunteers. J Clin Pharmacol. 2008;48:1171–8. doi: 10.1177/0091270008323753. [DOI] [PubMed] [Google Scholar]
- 2.Eckhardt M, Langkopf E, Mark M, Tadyyon M, Thomas L, Nar H, Pfrengle W, Guth B, Lotz R, Sieger P, Fuchs H, Himmelsbach F. 8-(3-(R)-aminopiperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydropurine-2,6-dione (BI 1356), a highly potent, selective, long-acting, and orally bioavailable DPP-4 inhibitor for the treatment of type 2 diabetes. J Med Chem. 2007;50:6450–3. doi: 10.1021/jm701280z. [DOI] [PubMed] [Google Scholar]
- 3.Thomas L, Eckhardt M, Langkopf E, Tadayyon M, Himmelsbach F, Mark M. (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action compared with other dipeptidyl peptidase-4 inhibitors. J Pharmacol Exp Ther. 2008;325:175–82. doi: 10.1124/jpet.107.135723. [DOI] [PubMed] [Google Scholar]
- 4.Campbell RK. Rationale for dipeptidyl peptidase 4 inhibitors: a new class of oral agents for the treatment of type 2 diabetes mellitus. Ann Pharmacother. 2007;41:51–60. doi: 10.1345/aph.1H459. [DOI] [PubMed] [Google Scholar]
- 5.Deacon CF, Ahrén B, Holst JJ. Inhibitors of dipeptidyl peptidase IV: a novel approach for the prevention and treatment of type 2 diabetes? Expert Opin Investig Drugs. 2004;13:1091–102. doi: 10.1517/13543784.13.9.1091. [DOI] [PubMed] [Google Scholar]
- 6.Langley AK, Suffoletta TJ, Jennings HR. Dipeptidyl peptidase IV inhibitors and the incretin system in type 2 diabetes mellitus. Pharmacotherapy. 2007;27:1163–80. doi: 10.1592/phco.27.8.1163. [DOI] [PubMed] [Google Scholar]
- 7.Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and non-diabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E119–206. doi: 10.1152/ajpendo.00545.2003. [DOI] [PubMed] [Google Scholar]
- 8.Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care. 2003;26:2929–40. doi: 10.2337/diacare.26.10.2929. [DOI] [PubMed] [Google Scholar]
- 9.Food and Drug Administration, HHS. International Conference on Harmonisation; guidance on E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. Fed Regist. 2005;70:61134–5. [PubMed] [Google Scholar]
- 10.Cubeddu LX. QT prolongation and fatal arrhythmias: a review of clinical implications and effects of drugs. Am J Ther. 2003;10:452–7. doi: 10.1097/00045391-200311000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Heise T, Graefe-Mody EU, Hüttner S, Ring A, Trommeshauser D, Dugi KA. Pharmacokinetics, pharmacodynamics and tolerability of multiple oral doses of linagliptin, a dipeptidyl peptidase-4 inhibitor in male type 2 diabetes patients. Diabetes Obes Metab. 2009;11:786–94. doi: 10.1111/j.1463-1326.2009.01046.x. [DOI] [PubMed] [Google Scholar]
- 12.Deacon CF, Holst JJ. Linagliptin, a xanthine-based dipeptidyl peptidase-4 inhibitor with an unusual profile for the treatment of type 2 diabetes. Expert Opin Investig Drugs. 2010;19:133–40. doi: 10.1517/13543780903463862. [DOI] [PubMed] [Google Scholar]
- 13.Forst T, Uhlig-Laske B, Ring A, Graefe-Mody U, Friedrich C, Herbach K, Woerle H-J, Dugi KA. Linagliptin (BI 1356), a potent and selective DPP-4 inhibitor, is safe and efficacious in combination with metformin in patients with inadequately controlled type 2 diabetes. Diabet Med. 2010;27:1409–19. doi: 10.1111/j.1464-5491.2010.03131.x. DOI: 10.1111/j.1464-5491.2010.03131.x. [DOI] [PubMed] [Google Scholar]
- 14.Del Prato S, Barnett AH, Huisman H, Neubacher D, Woerle H-J, Dugi KA. Effect of linagliptin monotherapy on glycaemic control and markers of β-cell function in patients with inadequately controlled type 2 diabetes: a randomised controlled trial. Diabetes Obes Metab. 2011;13:258–67. doi: 10.1111/j.1463-1326.2010.01350.x. DOI: 10.1111/j.1463-1326.2010.01350.x. [DOI] [PubMed] [Google Scholar]
- 15.Taskinen M-R, Rosenstock J, Tamminen I, Kubiak R, Patel S, Dugi KA, Woerle H-J. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13:65–74. doi: 10.1111/j.1463-1326.2010.01326.x. DOI: 10.1111/j.1463-1326.2010.01326.x. [DOI] [PubMed] [Google Scholar]
- 16.Gomis R, Espadero R-M, Jones R, Woerle H-J, Dugi KA. Efficacy and safety of initial combination therapy with linagliptin and pioglitazone in patients with inadequately controlled type 2 diabetes. Poster 551-P presented at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010, abstract available online. Available at http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=79499 (last accessed 4 November 2010) [DOI] [PubMed]
- 17.Owens DR, Swallow R, Jones P, Dugi KA, Woerle H-J. Linagliptin improves glycemic control in type 2 diabetes patients inadequately controlled by metformin and sulfonylurea without weight gain or hypoglycemia. Poster 548-P presented at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010, abstract available online. Available at http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=79496 (last accessed 4 November 2010) [DOI] [PubMed]
- 18.Morganroth J. A definitive or thorough phase 1 QT ECG trial as a requirement for drug safety assessment. J Electrocardiol. 2004;37:25–9. doi: 10.1016/j.jelectrocard.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Bloomfield DM, Krishna R, Hreniuk D, Hickey L, Ghosh K, Bergman AJ, Miller J, Gutierrez MJ, Stoltz R, Gottesdiener KM, Herman GA, Wagner JA. A Thorough QTc study to assess the effect of sitagliptin, a DPP4 inhibitor, on ventricular repolarization in healthy subjects. J Clin Pharmacol. 2009;49:937–46. doi: 10.1177/0091270009337511. [DOI] [PubMed] [Google Scholar]
- 20.Moss AJ, Morganroth J. Cardiac effects of ebastine and other antihistamines in humans. Drug Saf. 1999;21(Suppl 1):69–80. doi: 10.2165/00002018-199921001-00009. [DOI] [PubMed] [Google Scholar]
- 21.Noel GJ, Natarajan J, Chien S, Hunt TL, Goodman DB, Abels R. Effects of three fluoroquinolones on QT interval in healthy adults after single doses. Clin Pharmacol Ther. 2003;73:292–303. doi: 10.1016/s0009-9236(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 22.Jones B, Kenward MG. Design and Analysis of Cross-Over Trials. 2nd edn. Boca Raton, FL: CRC Press/Taylor and Francis Group; 2003. ISBN 0-412-60640-2. [Google Scholar]
- 23.Zhang J, Machado SG. Statistical issues including design and sample size calculation in thorough QT/QTc studies. J Biopharm Stat. 2008;18:451–67. doi: 10.1080/10543400802020938. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Silkey M, Schumacher M, Wang L, Raval H, Caulfield JP. Period correction of the QTc of moxifloxacin with multiple predose baseline ECGs is the least variable of 4 methods tested. J Clin Pharmacol. 2009;49:534–9. doi: 10.1177/0091270008330158. [DOI] [PubMed] [Google Scholar]
- 25.Glomb P, Ring A. Use of baseline ECGs in the evaluation of thorough-QT studies with crossover design. 54th Biometric Colloquium, Munich, 2008. Available at http://130.75.68.3/ibs/arbeitsgruppen/ag-lwv/tagungsberichte-1/2008_munchen/lifestat2008_abstract_band.pdf, page 233; http://www.biopharmnet.com/doc/2008_04_15_poster.pdf.
- 26.Ring A. Statistical models for heart rate correction of the QT interval. Stat Med. 2010;29:786–96. doi: 10.1002/sim.3791. [DOI] [PubMed] [Google Scholar]
- 27.Shah A, Hajain GA. Maximum likelihood approach for estimating the QT correction factor using mixed effects model. Stat Med. 2003;22:1901–9. doi: 10.1002/sim.1434. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Pan G, Balch A. Bias and variance evaluation of QT interval correction methods. J Biopharm Stat. 2008;18:427–50. doi: 10.1080/10543400801992988. [DOI] [PubMed] [Google Scholar]
- 29.Patel CG, Li L, Komoroski BJ, Frevert EU, Kornhauser DM, Boulton DW. No effect of saxagliptin on QTc Interval in healthy subjects. Abstract 2072-PO. Presented at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 05–09 June 2009. Abstract available online at http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=74185 (last accessed 4 November 2010)
- 30.European Medicines Agency (EMEA) Galvus (vildagliptin) – European Public Assessment Report (EPAR) – Scientific Discussion. 2007. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000771/WC500020330.pdf (last accessed 04 November 2010)
- 31.Malik M. Facts, fancies and follies of drug-induced QT/QTc interval shortening. Br J Pharmacol. 2009;159:70–6. doi: 10.1111/j.1476-5381.2009.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malik M, Hnatkova K, Batchvarov V. Differences between study-specific and subject-specific heart rate corrections of the QT interval in investigations of drug induced QTc prolongation. Pacing Clin Electrophysiol. 2004;27:791–800. doi: 10.1111/j.1540-8159.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 33.Schall R, Ring A. Statistical characterisation of QT prolongation. J Biopharm Stat. 2010;20:543–62. doi: 10.1080/10543400903581978. [DOI] [PubMed] [Google Scholar]
- 34.Schall R, Ring A. Mixed models for data from thorough QT studies. Part 1. Assessment of marginal QT prolongation. Pharm Stat. 2011 doi: 10.1002/pst.463. DOI: 10.1002/pst.463. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Smith B. Sex differences in QT interval variability and implication on sample size of thorough QT study. Drug Inf J. 2007;41:619–27. [Google Scholar]
- 36.Beasley CM, Dmitrienko A, Mitchell MI. Design and analysis considerations for thorough QT studies employing conventional (10 s, 12-lead) ECG recordings. Exp Review Clin Pharmacol. 2008;1:815–39. doi: 10.1586/17512433.1.6.815. [DOI] [PubMed] [Google Scholar]
- 37.Démolis JL, Kubitza D, Tennezé L, Funck-Brentano C. Effect of a single oral dose of moxifloxacin (400 mg and 800 mg) on ventricular repolarization in healthy subjects. Clin Pharmacol Ther. 2000;68:658–66. doi: 10.1067/mcp.2000.111482. [DOI] [PubMed] [Google Scholar]


