The von Hippel-Lindau (VHL) gene plays a central role in the pathogenesis of both hereditary and sporadic renal cell carcinoma (RCC). VHL encodes a component of an E3 ubiquitin ligase complex that degrades hypoxia inducible transcription factors (HIFs) HIF-1α and HIF-2α in an oxygen-dependent manner. The loss of VHL therefore results in aberrant HIF stabilization under normal oxygen tension. HIF-2α stabilization is a key step in the pathogenesis of renal cell carcinoma;1 however, the range of downstream target genes responsible for HIF dependent tumorigenesis remains to be fully elucidated. HIF targets that have been implicated in renal cancer include angiogenic factors (VEGF, PDGF), mitogens (EGF-R and its ligand TGFα), as well as glycolytic enzymes (GLUT1, LDH-A). Identification of these downstream targets has led to the emergence of targeted therapy for treating advanced RCC, including the use of sorafenib, sunitinib and bevacizumab to block VEGF signaling, and sirolimus to inhibit the mTOR pathway.2,3
Several groups have recently shown that HIFs can modulate c-MYC transcriptional activity in renal cancer cells via multiple mechanisms. First, HIF-1α antagonizes c-MYC transcriptional activity to cause cell cycle arrest and genomic instability.4,5 Second, HIF-2α enhances the MYC-MAX interaction to promote c-MYC dependent cell cycle progression.6 Third, HIF-1α and HIF-2α induce MXI1 expression to suppress mitochondrial biogenesis and c-MYC dependent apoptosis.7,8
The c-Myc oncogene is a basic loop-helix-loop-zipper (bHLHZ) transcription factor that binds E-box DNA sequences in conjunction with its partners Max and Mad. The Mad family of proteins consists of MAD1, MXI1, MAD3 and MAD4. While Myc-Max dimers activate transcription, the binding of Mad-Max dimers—including MXI1-MAX—to promoter E-boxes leads to transcriptional repression due to the ability of Mad proteins to recruit histone deacetylases as co-repressors.9 This ability of Mad family proteins to inhibit c-Myc dependent transcription is consistent with their putative roles as tumor suppressors. For example, Mxi1 knockout mice are more prone to developing cancer and embryonic fibroblasts derived from these mice undergo Ras-Myc transformation more efficiently compared to wild type control.10 However, the role of MXI1 in tumorigenesis remains unclear because of conflicting reports on MXI1 mutations and its expression in primary human malignancies.11-13
It was recently discovered that MXI1 is a direct HIF target gene that regulates the metabolic profile of RCC cells.8 In this issue of Cancer Biology & Therapy, Tsao et al.14 provide further evidence for the oncogenic role of MXI1 in renal cell carcinoma. The authors first observed that human RCC tissues overexpress MXI1 in a pattern identical to that of HIF-2α expression. They then used short hairpin RNA (shRNA) to deplete MXI1 in 786-O cells, an RCC derived cell line that expresses HIF-2α exclusively. Although MXI1 knockdown increased proliferation in vitro, it significantly impaired xenograft formation in nude mice. This discrepancy between MXI1 knockdown phenotypes in culture and in mice is consistent with the observation that HIF-2α inhibition in these cells does not limit proliferation in vitro, but is sufficient to impair xenograft growth.15,16 The introduction of a plasmid encoding shRNA-resistant MXI1 into 786-O cells rescued the effect of MXI1 knockdown on xenograft formation, indicating a specific role for MXI1 in RCC tumorigenesis.
To determine the mechanism behind MXI1 effects on tumorigenesis, Tsao et al.14 showed by immunohistochemistry that MXI1 suppressed xenograft growth by inhibiting proliferation, while caspase-dependent apoptosis was unaffected. Although MXI1 overexpression has been demonstrated to protect cells from hypoxia-induced apoptosis, especially in the context of increased c-MYC activity and growth factor deprivation,7 this response does not seem to predominate in RCC xenografts. Supporting this argument, the expression level of ornithine decarboxylase (ODC), which is involved in c-MYC dependent apoptosis,17 was unaffected by MXI1 knockdown in 786-O cells. Because MXI1 regulates mitochondrial biogenesis in RCC cells via the c-MYC target gene PGC-1β,8 the authors next investigated the effect of depleting MXI1 on PGC-1β expression. Transcript levels of PGC-1β were unchanged in 786-O cells lacking MXI1, indicating that the effect of MXI1 knockdown on xenograft growth is unlikely to be due to increased mitochondrial respiration and ROS production.
Apart from c-MYC induced apoptosis, other c-MYC dependent pathways, such as cell proliferation and differentiation, are unlikely to account for the growth impairment observed in RCC xenografts lacking MXI1. The authors thus hypothesize that MXI1 may be acting independently of c-MYC to promote tumorigenesis. Previous work has shown that HIF-1α antagonizes while HIF-2α enhances c-MYC transcriptional activity.4-6 However, the findings of Tsao et al.14 predict that HIF-2α induction of MXI1 will inhibit c-Myc dependent transcription. How can these two contradictory views be reconciled? One possible explanation is that the effects of MXI1 activity are dependent on HIF protein status. For example, in the presence of HIF-2α alone, MXI1 may be functionally inactive because of HIF-2α enhanced dimerization of Myc and Max. In contrast, when HIF-1α and HIF-2α are stabilized, presumably both HIF-1α and MXI1 will promote the formation of repressive Mad-Max complexes. The data presented by Tsao et al.14 are consistent with this model. In 786-O cells, which express HIF-2α exclusively, MXI1 suppression did not affect c-MYC target gene expression. Conversely, in RCC4 cells, which express both HIF-1α and HIF-2α, MXI1 knockdown resulted in the upregulation of both PGC-1β and ODC1. Although Tsao et al.14 were unable to establish xenografts using the RCC4 cell line, it will be of interest in the future to determine whether MXI1 depletion impairs the growth of VHL deficient tumors expressing both HIF-1α and HIF-2α.
The anti-tumor effect of MXI1 knockdown in 786-O xenografts is strikingly similar to that achieved with HIF-2α suppression in a 786-O subclone.1 This result suggests that the inhibition of MXI1 is sufficient to impair HIF-2α driven tumorigenesis. Given that hypoxia is a characteristic of many cancers, it will also be important to test whether MXI1 is induced by HIF-1α or HIF-2α in VHL proficient tumors, and whether their growth can be slowed by the suppression of MXI1.
Is MXI1 a tumor suppressor or oncogene? Previous work had suggested a role for Mxi1 in suppressing tumorigenesis.10 Tsao et al.14 now demonstrate that MXI1 inhibition suppresses the growth of RCC xenografts. It is possible that MXI1 can act as either oncogene or tumor suppressor depending on the cellular context. In support of this view, MXI1 regulates c-MYC, which is known to exhibit pro-oncogenic or anti-oncogenic properties depending on molecular and cellular context. Although the mechanisms by which MXI1 contribute to the RCC phenotype remain to be uncovered, the findings by Tsao et al.14 extend our knowledge of the HIF transcription network and identify MXI1 and its downstream effectors as potential therapeutic targets in renal cell cancer.
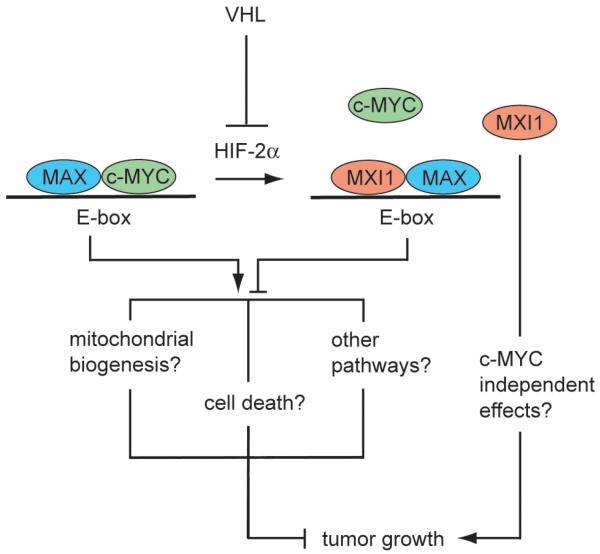
Figure 1. Model for MXI1 regulation of VHL deficient RCC tumorigenesis.
The loss of VHL in RCC leads to normoxic stabilization of HIF-1α and HIF-2α. HIF-2α (and HIF-1α) induces the expression of MXI1, which binds to the c-MYC binding protein MAX and thus displaces c-MYC from the transcriptionally active Myc-Max complex. The binding of MXI1-MAX heterodimers to DNA E-boxes antagonizes c-MYC dependent transcription, resulting in enhanced tumorigenesis via a number of possible mechanisms; MXI1 may also exert c-MYC independent effects on cancer development.
Footnotes
Commentary to: Tsao CC, Teh BT, Jonasch E, Schreiber-Agus N, Efstathiou E, Hoang A, Czerniak B, Logothetis C, Corn PG. Inhibition of Mxi1 suppresses HIF-2α-dependent renal cancer tumorigenesis. Cancer Biol Ther 2008; This issue.
References
- 1.Kondo K, Kim WY, Lechpammer M, Kaelin WG., Jr. Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1:439–44. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, Ginsberg MS, Bacik J, Kim ST, Baum CM, Michaelson MD. Sunitinib in patients with metastatic renal cell carcinoma. Jama. 2006;295:2516–24. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 3.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O’Toole T, Lustgarten S, Moore L, Motzer RJ. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 4.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–56. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koshiji M, To KK, Hammer S, Kumamoto K, Harris AL, Modrich P, Huang LE. HIF-1alpha induces genetic instability by transcriptionally downregulating MutSalpha expression. Mol Cell. 2005;17:793–803. doi: 10.1016/j.molcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–47. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corn PG, Ricci MS, Scata KA, Arsham AM, Simon MC, Dicker DT, El-Deiry WS. Mxi1 is induced by hypoxia in a HIF-1-dependent manner and protects cells from c-Myc-induced apoptosis. Cancer Biol Ther. 2005;4:1285–94. doi: 10.4161/cbt.4.11.2299. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–20. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 9.SchreiberA-gus N, Chin L, Chen K, Torres R, Rao G, Guida P, Skoultchi AI, DePinho RA. An amino-terminal domain of Mxi1 mediates anti-Myc oncogenic activity and interacts with a homolog of the yeast transcriptional repressor SIN3. Cell. 1995;80:777–86. doi: 10.1016/0092-8674(95)90356-9. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber-Agus N, Meng Y, Hoang T, Hou H, Jr, Chen K, Greenberg R, Cordon-Cardo C, Lee HW, DePinho RA. Role of Mxi1 in ageing organ systems and the regulation of normal and neoplastic growth. Nature. 1998;393:483–7. doi: 10.1038/31008. [DOI] [PubMed] [Google Scholar]
- 11.Kuczyk MA, Serth J, Bokemeyer C, Schwede J, Herrmann R, Machtens S, Grunewald V, Hofner K, Jonas U. The MXI1 tumor suppressor gene is not mutated in primary prostate cancer. Oncol Rep. 1998;5:213–6. [PubMed] [Google Scholar]
- 12.Ariyanayagam-Baksh SM, Baksh FK, Swalsky PA, Finkelstein SD. Loss of heterozygosity in the MXI1 gene is a frequent occurrence in melanoma. Mod Pathol. 2003;16:992–5. doi: 10.1097/01.MP.0000087421.44975.1C. [DOI] [PubMed] [Google Scholar]
- 13.Boult JK, Taniere P, Hallissey MT, Campbell MJ, Tselepis C. Oesophageal adenocarcinoma is associated with a deregulation in the MYC/MAX/MAD network. Br J Cancer. 2008;98:1985–92. doi: 10.1038/sj.bjc.6604398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsao CC, Teh BT, Jonasch E, Schreiber-Agus N, Efstathiou E, Hoang A, Czerniak B, Logothetis C, Corn PG. Inhibition of Mxi1 suppresses HIF-2α-dependent renal cancer tumorigenesis. Cancer Biol Ther. 2008;7:1619–27. doi: 10.4161/cbt.7.10.6583. [DOI] [PubMed] [Google Scholar]
- 15.Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG., Jr. Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–46. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 16.Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–86. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Packham G, Cleveland JL. Ornithine decarboxylase is a mediator of c-Myc-induced apoptosis. Mol Cell Biol. 1994;14:5741–7. doi: 10.1128/mcb.14.9.5741. [DOI] [PMC free article] [PubMed] [Google Scholar]