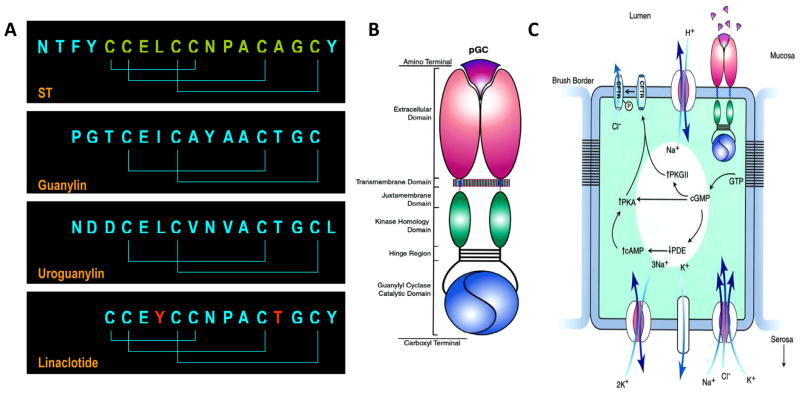
Figure 1.
Guanylyl cyclase C ligands and intestinal secretion. (A) Homologous peptide ligands that activate GCC include the diarrheagenic bacterial heat-stable enterotoxins (ST) and the paracrine hormones guanylin, produced in the colon, and uroguanylin, produced in the small intestine. Linaclotide is a substituted (red residues) ST analog preserving the 3 disulfide bonds delimiting the 13 amino acid biologically active core ST peptide (green) required for maximum activity and stability. (B) GCC is one isoform of the seven member (GCA - GCG) subfamily of membrane-bound particulate guanylyl cyclases (pGC) which are homo-oligomers comprising extracellular ligand binding, short transmembrane, cytoplasmic juxtamembrane, regulatory kinase homology, oligomerization (hinge), and catalytic domains. (C) Regulation of intestinal secretion by GCC.6 Cognate peptides in the intestinal lumen ligate the extracellular domain of GCC, expressed in brush border membranes of epithelial cells. Ligand-receptor interaction is translated across the plasma membrane into activation of the cytoplasmic catalytic domain producing accumulation of intracellular cyclic GMP. This cyclic nucleotide directly activates cyclic GMP or cyclic AMP-dependent protein kinase (PKG or PKA, respectively). Also, cyclic GMP inhibits cyclic AMP-specific phosphodiesterase (PDE), increasing intracellular cyclic AMP concentrations, which can activate PKA. The ultimate result of cyclic GMP accumulation is phosphorylation of the cystic fibrosis transmembrane conductance regulator (CFTR) which is co-localized with GCC in brush border membranes. CFTR is a chloride channel and its phosphorylation by protein kinase results in a persistent open state, permitting chloride to flow down a concentration gradient from intracellular to the extracellular compartments. Other ion channels and transporters maintain electroneutrality of cyclic GMP-dependent chloride efflux. Vectoral water flux from basolateral to the apical membranes is driven by these ionic conductances, resulting in accumulation of fluid and electrolytes in the intestine and, in the case of STs, secretory diarrhea.