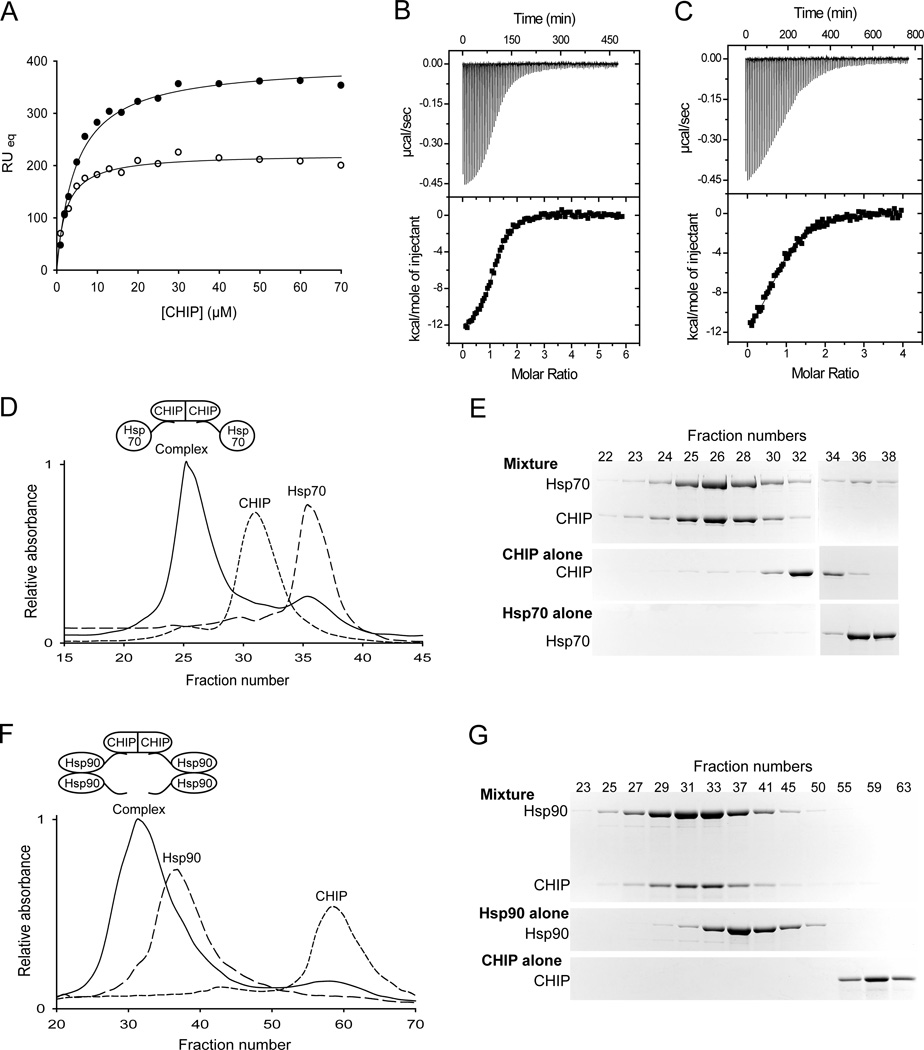
FIGURE 2.
Complex formation between Hsp70 or Hsp90 and CHIP. (A) CHIP-peptide interactions monitored by surface plasmon resonance (SPR). Plot of the average response at equilibrium (RUeq) versus concentration of CHIP. Open circles show binding to immobilized C-terminal peptide of Hsp70, solid circles show binding to immobilized C-terminal peptide of Hsp90. The solid lines show fits to a one-site binding equation, with dissociation constants of ~ 2 µM and ~ 5 µM, for CHIP binding to Hsp70 and Hsp90, respectively. (B and C) Isothermal Titration Calorimetry (ITC). Upper panels: thermogram of the titration of the C-terminal peptide of Hsp70 (B) or Hsp90 (C) into a solution of CHIP. Lower panels: plot of integrated areas under the peaks of heat in the upper panel as a function of molar ratio. Fitting the data gives a dissociation constant of ~ 1 µM with a stoichiometry of ~ 0.8 C-terminal Hsp70 peptide per CHIP monomer and a dissociation constant of ~ 4.4 µM with a stoichiometry of ~ 0.9 Hsp90 C-terminal peptide per CHIP monomer. (D – G) Complex formation between CHIP and Hsp70 or CHIP and Hsp90, all full-length. Size-exclusion chromatograms of purified CHIP dimer alone, purified Hsp70 monomer alone and an equimolar mixture of the two proteins (D). Coomassie-stained SDS-polyacrylamide gels of the indicated fractions (E). Size-exclusion chromatography analysis of purified CHIP dimer alone, purified Hsp90 dimer alone and an equimolar mixture of the two proteins (F). Coomassie-stained SDS-polyacrylamide gels of the indicated fractions (G). Cartoons of the proposed CHIP-Hsp70 and CHIP-Hsp90 complexes, based on the analysis of the composition of each fraction (see experimental procedures), are shown in (D) and (F), respectively. CHIP does not elute in the same fractions in (D) and (F), because the volume of the fractions is different in the two experiments.