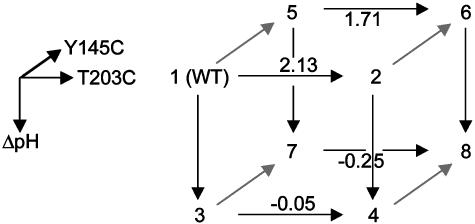
Fig. 6.
An experimental test of the prediction of three-way independence of T203C, Y145C, and ΔpH. By extension from the definition of the two-way coupling energy (see text), the three-way coupling is the degree to which a given two-way coupling between two mutations depends on a third mutation. A schematic representation of this is the thermodynamic cube, where the front face is the double mutant cycle for T203C–ΔpH, and the back face the same cycle in the background of Y145C. The difference in the two-way coupling energies of the front and back faces gives the three-way coupling energy of the mutants (ΔΔΔGT203C,Y145C, ΔpH = 0.22 ± 0.29 kcal/mol). The cube vertices represent: 1, WT, pH 8.5; 2, T203C, pH 8.5; 3, WT, pH 5.5; 4, T203C, pH 5.5; 5, Y145C, pH 8.5; 6, Y145C, T203C, pH 8.5; 7, Y145C, pH 5.5; 8, Y145C, T203C, pH 5.5. Values shown above arrows are difference energies for the associated pair of mutants in kcal/mol.