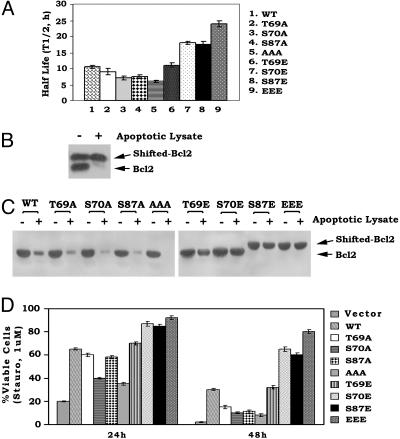
Fig. 6.
Multisite phosphorylation of Bcl2 induced by either paclitaxel or substitution of glutamate can enhance Bcl2's stability and resistance to proteolysis. (A) Cells expressing WT or various A- or E-Bcl2 mutants were metabolically labeled with [35S]methionine. The t1/2 of WT and mutant Bcl2 was determined by classic pulse–chase analysis. (B and C) Mobility-shifted Bcl2 was immunoprecipitated from lysates of cells expressing WT Bcl2 after treatment with paclitaxel to induce multisite Bcl2 phosphorylation. A- and E-containing Bcl2 mutants were immunoprecipitated from lysates of untreated cells expressing A- or E-mutants. These Bcl2 immune complexes were then incubated with an apoptotic extract for 2 h, and Bcl2 was analyzed by Western blotting by using Bcl2 antibody. (D) NSF.N1/H7 cells expressing equivalent amounts of WT or A- or E-Bcl2 mutants were treated with 1 μM staurosporine for 24 and 48 h. Cell viability was assessed as described for Fig. 1D. Data represent the mean ± SD of three independent determinations.