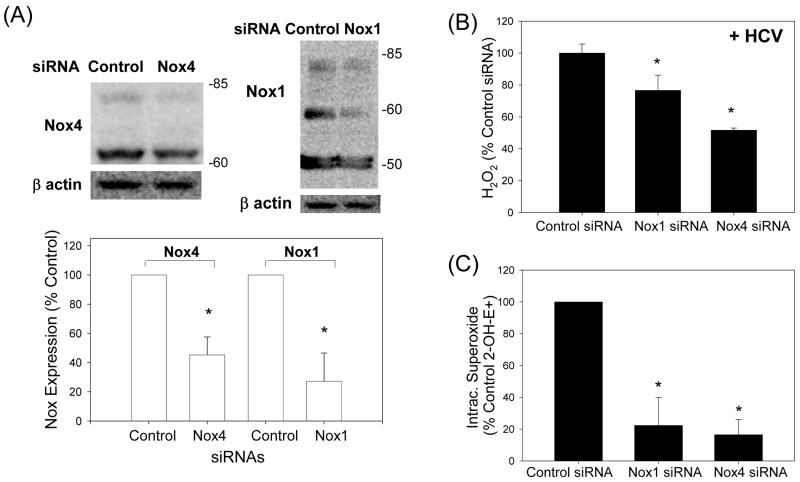
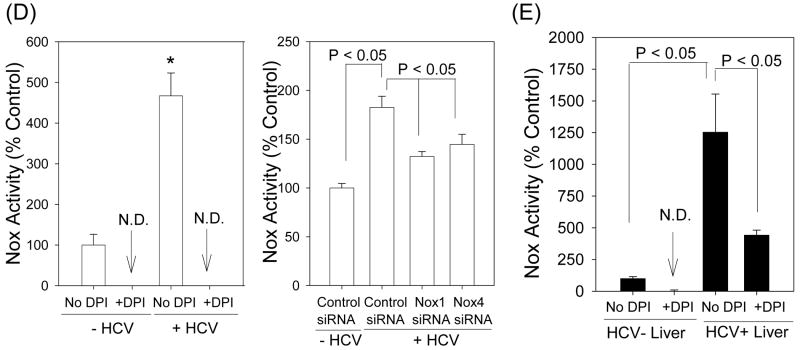
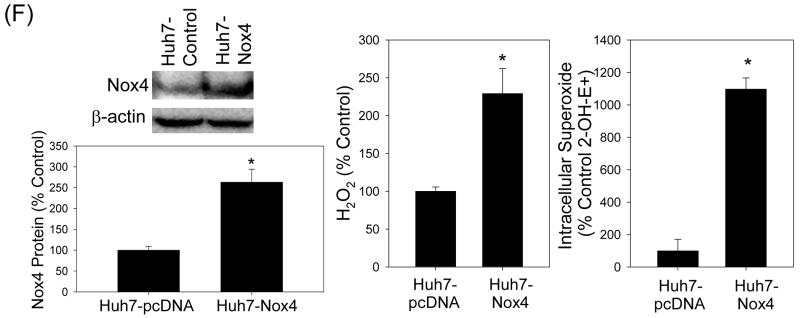
Fig. 4. Role of Nox enzymes in HCV-induced ROS.
(A – C) Twenty four hrs after JFH1 RNA transfection, Huh7 cells were transfected with non-targeting control siRNA, Nox1 siRNA, or Nox4 siRNA and, after another 72 hrs, analyzed for the level of Nox1 and Nox4 proteins by western blots (A), H2O2 by p-hydroxyphenylaminoacetic acid dimerization assay (B), and intracellular superoxide by measuring 2-OH-E+ via HPLC (C). (D) Cells were permeabilized with intracellular-like buffer containing 40 μM digitonin, and Nox enzyme activities were determined by monitoring NADPH-dependent and SOD-inhibited reduction of cytochrome c, in the presence and absence of 30 μM DPI, as described in the Supplement. Activity assays were also carried out after transfecting cells with non-targeting control siRNA, Nox1 siRNA, or Nox4 siRNA. (E) Human liver samples were sonicated in intracellular-like buffer, and SOD-inhibited reduction of cytochrome c was determined in the presence and absence of DPI, as described in the Supplement. (F) Huh7 cells stably transfected with Nox4 cDNA or empty plasmid vector (pcDNA) alone were analyzed for Nox4 protein, H2O2, and superoxide, as described. Nox proteins were quantified by densitometry and normalized by β-actin. Data in (B - F) were normalized by total protein. * indicates statistically significant difference from controls (P < 0.05). N.D. indicates “non-detectable” (below baseline).