Figure 2.
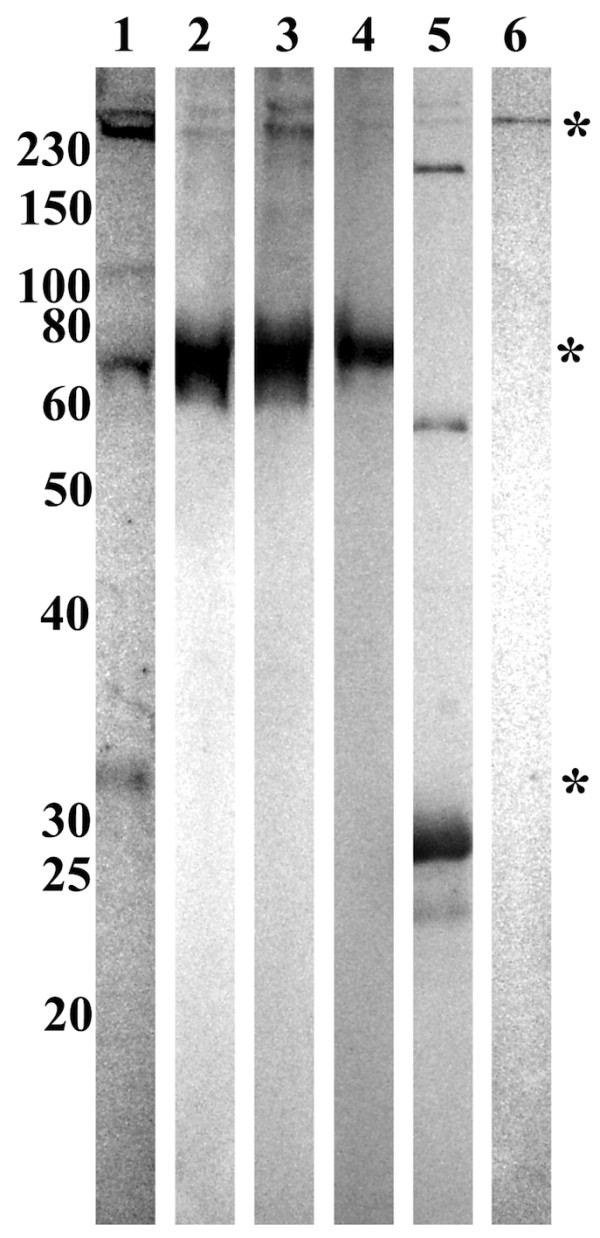
Immunoblotting of tick haemolymph proteins with anti-ficolin antibodies. Electrophoretically separated and electroblotted non-reduced haemolymph proteins from D. marginatus (lane 1), R. appendiculatus (lane 2), R. pulchellus (lane 3), and R. sanguineus (lane 4) were immunostained using rabbit anti-FCN1 H antibodies. Recombinant human ficolin 1 was used as a control (lane 5). Purified hemelipoglycoprotein from D. marginatus haemolymph, which was identified by MS as one of the recognised proteins was used as a control (lane 6). However, this protein does not contain the fibrinogen domain [3]. The same proteins as in Figure 1A were detected in D. marginatus haemolymph (36 kDa, 79/80 kDa, and 290 kDa proteins; marked with asterisks; the 79/80 kDa double-band is marked by one asterisk). In Rhipicephalus ticks haemolymphs, the 72 kDa and 290 kDa proteins were detected, but not the 55 kDa protein. Additionally, the purified hemelipoglycoprotein from D. marginatus was detected by the anti-FCN1 H antibodies. Non-reduced recombinant human ficolin 1 served as a positive control. Antibodies positively reacted with subunits of the protein (approximately 30 kDa) as well as with higher molecular weight complexes (approximately 60 kDa, 180 kDa, 250 kDa, 280 kDa).
