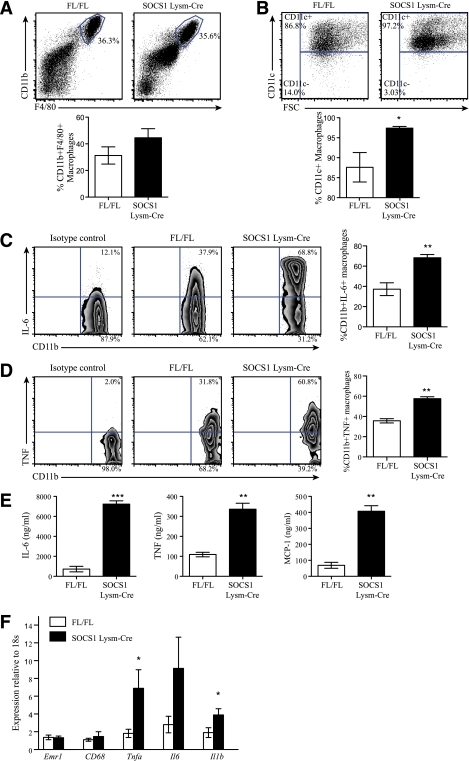
FIG. 2.
Macrophages from SOCS1 LysM-Cre mice are constitutively activated and inflamed. Peritoneal cells were isolated and stained with antibodies against F4/80, CD11b, CD11c, or isotype controls and analyzed by flow cytometry. A: Number of F4/80+CD11b+ cells in wild-type and SOCS1 LysM-Cre mice indicated as percentage of viable peritoneal cell population. B: Cells were gated for F4/80+CD11b+ cells and examined for expression of CD11c. Shown is the percentage of CD11c-positive or -negative cells within the total F4/80+CD11b+ population. A and B: Top panels are representative plots; bottom panels are means ± SEM, n = 8 per group. White bars, FL/FL control mice; black bars, SOCS1 LysM-Cre mice. Representative plots of the intracellular cytokine staining to detect secretion of IL-6 (C) and TNF-α (D) in peritoneal macrophages are shown. For each, cells were gated on the CD11b+ population, and data represented in the graph are combined from two experiments and expressed as mean ± SEM; n = 6 per group. E: Quantitation of cytokine secretion by CD11b+ peritoneal macrophages from littermate control and SOCS1 LysM-Cre mice. Secretion of IL-6, TNF, and MCP-1 by CD11b+ peritoneal macrophages was measured by cytokine bead array. F: Adipose tissue expression of Emr1, Cd68, Tnfa, Il6, and Il1b in SOCS1 LysM-Cre and control mice. Data are shown as mean ± SEM; n = 8 per group. White bars, FL/FL control mice; black bars, SOCS1 LysM-Cre mice. For all experiments, *P < 0.05, **P < 0.01, and ***P < 0.001 relative to control. (A high-quality color representation of this figure is available in the online issue.)