Figure 6. Competitive analysis (NoShift assay) of R9-Nanog (A), R9-Oct3/4 (B), R9-Sox2 (C) showing that nona-arginine fusion proteins exhibit cognate DNA binding activity.
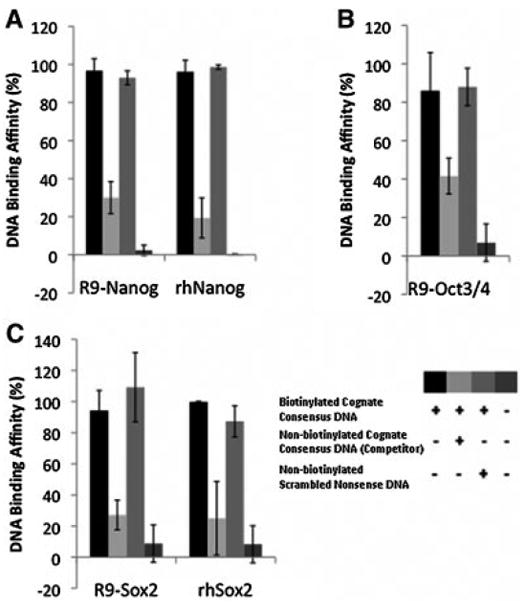
When incubated with a biotinylated cognate consensus sequence, R9-fusion protein-DNA binding was observed. Specific non-biotinylated competitor DNA with the cognate consensus sequence significantly reduced the binding activity in each R9-fusion protein, confirming sequence-specificity of the assay for R9-fusion protein binding. Non-biotinylated scrambled nonsense sequences had no effect on R9-fusion protein binding. As a positive control, human recombinant proteins (i.g. rhNanog and rhSox2) were used.
