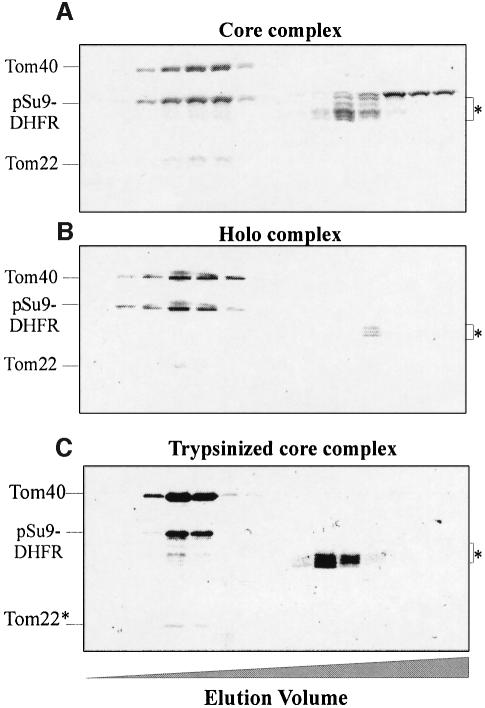
Fig. 2. Binding of mitochondrial precursor protein to isolated soluble TOM complex analyzed by size-exclusion chromatography. TOM core complex (84 µg) (A) and holo complex (122 µg) (B) were incubated for 30 min at 4°C with pSu9(1–69)-DHFR (202 and 245 µg, respectively). The mixtures were then loaded on a size-exclusion column. Proteins were eluted, precipitated with trichloroacetic acid and analyzed by SDS–PAGE and Coomassie staining. Bands corresponding to the TOM components and the precursor protein are indicated. The preparations of precursor protein contained some degradation products (marked with an asterisk), which eluted in the lower molecular weight range. (C) Binding of pSu9(1–69)-DHFR to trypsinized TOM core complex. Purified TOM core complex (30 µg) was treated with trypsin, re-isolated by gel filtration and incubated for 30 min at 4°C with 60 µg of pSu9(1–69)-DHFR. Further treatment was as described above. Tom22*, trypsin-resistant fragment of Tom22.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.