Abstract
Recent studies have reported that reproductive experience in female rats alters prolactin (PRL) receptor gene expression in the brain as well as neural sensitivity to PRL. Given PRL's actions in nonneural tissues, that is, mammary tissue and liver, it was asked whether reproductive experience may also alter prolactin receptor (Prlr) gene expression in these tissues. Groups of age-matched female rats were generated with varying reproductive histories. Separate groups of primiparous (first lactation) and multiparous (second lactation) had mammary tissue and liver samples collected on Day 3 or 10 of lactation. A fifth group raised one litter to weaning and then resumed estrous cyclicity. This group and a final group of age-matched, virgin controls were killed on diestrus. Tissue was processed by quantitative PCR for expression rates of the long and short forms of Prlr mRNA as well as casein beta mRNA (mammary tissue only). Western blots were performed to quantify receptor protein content. Multiple lactations as well as lactation itself resulted in alterations in Prlr expression. Prlr gene expression in mammary tissue was increased in primiparous mothers compared with that in multiparous dams, whereas in the liver, Prlr expression was reduced during an initial lactation. In contrast, PRLR protein levels declined during lactation in mammary, but not hepatic, tissues. Overall, the results demonstrate that the prolactin receptor system is altered in nonneural tissues as a result of the female's reproductive history. The findings are discussed in the context of milk and bile production and PRL's possible role in breast cancer.
Keywords: lactation, liver, mammary glands, pregnancy, prolactin/prolactin receptor, reproductive experience
Prolactin receptor expression in the female rat is altered in mammary and hepatic tissues as a consequence of reproductive experience.
INTRODUCTION
The pituitary hormone prolactin (PRL) plays a key role in the physiological adaptations of pregnancy and lactation that prepare the female for energetic and behavioral demands postpartum [1–3]. Key roles that PRL plays include modulating stress responsivity during pregnancy and lactation [4], inducing the onset of maternal care [5], and stimulating lactogenesis [6]. Earlier research has shown that reproductive experience results in reductions in circulating levels of PRL in both women [7] and female rats [8, 9]. The functional significance of these reductions in circulating hormone is not known. However, it may involve an actual increase in responsiveness to PRL at the receptor level. In agreement with this concept are the findings that a long-term increase in the expression of the long form of the prolactin receptor (PRLRL) as well as its responsiveness to exogenous PRL treatment occur in the brain of experienced female rats after normal estrous cyclicity resumes [10]. Likewise, more recent research has found that basal as well as PRL-stimulated phosphorylated STAT5 activities in the brain are enhanced in reproductively experienced female rats [11].
In addition to PRL's actions within the brain, PRL acts on a number of key peripheral target tissues that include the mammary gland [1, 12] and liver [13, 14]. Primary functions of PRL in these tissues involve the stimulation of lactogenesis in mammary tissue [6] and the facilitation of bile transport in the liver [15]. Given that PRL acts at these nonneural sites, it was asked whether reproductive experience might produce shifts in PRLR expression in mammary and hepatic tissues as it does in selected neural sites. Earlier studies have demonstrated that prior parity can alter the responsiveness of mammary tissue to hormonal challenges [16] as well as result in changes in estrogen receptor expression [17]. Therefore, in the present study the effects of reproductive experience on the expression of both the long (PrlrL) and short (PrlrS) forms of the receptor [18] were measured by real-time RT-PCR in mammary tissue and liver in female rats that had varying amounts of reproductive experience. Moreover, receptor protein content was measured by Western blot analysis. Specifically, PRLR expression and content were compared between age-matched rats during an initial versus a second lactation and in nonlactating rats that had experienced one prior pregnancy and lactation or were inexperienced virgins. Finally, in order to compare possible changes in mammary function, expression of the milk protein, casein beta [19], was measured in these same treatment groups to see whether reproductive experience modified expression of this PRL-regulated protein.
MATERIALS AND METHODS
Animals
Fifty nulliparous Sprague-Dawley (Crl:CD[SD]BR) females (176–200 g) were purchased from Charles River Breeding Laboratories and triple-housed (45 × 25 × 20 cm polypropylene cages) in a light-controlled (lights on 0500–1900) and temperature-controlled (21°C–24°C) room with food and water available ad libitum throughout the current study. All the rats were maintained in accordance with the guidelines of the Division of Teaching and Research Resources at Tufts University, Cummings School of Veterinary Medicine (AAALAC accredited) following the procedures for animal care prepared by the National Research Council Committee of the Care and Use of Laboratory Animals Resources. The animal protocol (G911-07) was approved by Tufts University Institutional Animal Care and Use Committee. Twenty-five nulliparous females were mated with breeding males from our colony 3 wk after arrival at approximately 10 wk of age. On the day following parturition, each litter was culled to five males and five females. Females were allowed to raise their litters until weaning at 21 days postpartum. Approximately 11 wk after primiparous, females weaned their first litter (28 wk of age); 17 of these primiparous females together with 17 nulliparous females were mated with colony males to generate lactating multiparous and primiparous dams. The liver and mammary tissues were harvested from these rats at one of two points: D3 (Day 3) or D10 of lactation. The remaining nulliparous and primiparous females served as nonlactating controls. Vaginal smears were taken daily from both groups of nonlactating controls to characterize the individual estrous cycles. Tissue samples were collected from these subjects on the day of diestrus of the estrous cycle. Thus, the six treatment groups consisted of age-matched, nulliparous diestrous (N-Di), primiparous diestrous (P-Di), lactating primiparous D3 (P-D3) and D10 (P-D10), and lactating multiparous D3 (M-D3) and D10 (M-D10) subjects.
Tissue Collection
All the tissues were collected between 0900 and 1100 h on the designated collection day. After the animals were killed, the left lateral lobe of the liver together with the inguinal and abdominal mammary glands from one side were removed and kept frozen on dry ice under RNase-free conditions.
RNA Extraction and cDNA Synthesis
Total RNA was extracted from 100 mg of both mammary and liver tissues using TRI Reagent (Ambion) according to the manufacturer's instructions. Total RNA was measured using a NanoDrop ND-1000 spectrophotometer. M-MLV Reverse Transcriptase (Invitrogen) was used to make first strand cDNA from 200 ng of total RNA from each sample.
Real-Time PCR
TaqMan Gene Expression assays were purchased from Applied Biosystems for the long (Rn01525470_ml, RefSeq NM_001034111) and short (Rn01525458_ml, RefSeq NM_012630.1) forms of the rat Prlr as well as the casein beta gene (Rn00567460_ml, RefSeq NM_017120.2). Actin beta (Actb) was used as the housekeeping gene (Rn00667869_m1, RefSeq NM_031144.2; Applied Biosystems), and each sample was run in duplicate. For liver samples, TaqMan gene expression assays for both the long and short forms of Prlr as well as the Actb housekeeping gene were run simultaneously, and for the mammary tissue, the casein beta assay was included. Two animals from the nulliparous diestrous group were randomly selected to serve as controls and were included in all the reaction plates. RT-PCR was performed on an Applied Biosystems 7500 Real-Time PCR System. To calculate relative expression, ΔCt was calculated by subtracting the Ct (cycle threshold) of the housekeeping gene from each target. This calculation [20] was also used to determine the difference in Ct expression between the housekeeping and target genes from the mean Ct level from the two controls included in each plate (mean Ct target minus mean Ct housekeeping). The ΔΔCt was calculated by subtracting ΔCt control from ΔCt target. Finally, relative expression was calculated using theformula 2−ΔΔCt.
Western Blot Analyses
Individual samples of 100 mg of mammary or hepatic tissues were homogenized in 1 ml of PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4) prior to centrifugation; 10% of the homogenate was used to measure total protein content with a Coomassie Plus Protein Assay kit (Pierce Biotechnology). Dilutions of liver (5 μg/μl) and mammary (1 μg/μl) homogenates were taken, and equal volumes of sample, lysis buffer (catalog no. 89900; Thermo Scientific), and loading buffer (catalog no. 161-0737; Bio-Rad) were boiled for 5 min. These samples (15 μl/well) were loaded on 12% Tris-HCl gels together with molecular weight markers (37.8, 85.1, and 127.6 kDa). Gels were run at 90 V for approximately 3.5–4 h in running buffer (catalog no. 161-0771; Bio-Rad) and transferred to a polyvinylidene fluoride membrane at 33 V for 2 h in transfer buffer (catalog no. LC3675; Invitrogen).
The membrane was placed in 5% skim milk and 3% bovine serum albumin (BSA) for 1 hr prior to adding the primary antibody, mouse anti-PRLR U-5 (diluted 1:1000; all antibodies were purchased from Abcam), in 1% skim milk and 0.6% BSA overnight. This primary antibody binds to the extracellular domain of the PRLR and therefore recognizes both the long and short isoforms of the receptor. The second antibody, goat anti-mouse horseradish peroxidase (HRP)-conjugated immunoglobulin G (IgG) (diluted 1:10 000 in 1% skim milk and 0.6% BSA), was added for 1 h followed by ECL Western blot detection reagent (40A:1B) for 5 min; the membrane was exposed to film and the Restore Stripping Buffer (Pierce/Thermo Scientific) for 15 min. The membrane was subsequently placed in 5% skim milk for 1 h, and a primary antibody, goat anti-actin (diluted 1:200 in 1% skim milk) was added for 1 h followed by the addition of a secondary antibody, rabbit anti-goat HRP-conjugated IgG (diluted 1:10 000 in 1% skim milk), for 1 hr. The membrane was then exposed to 40A:1B for 5 min prior to being exposed to film.
The film was then scanned, and gel intensities were determined using a Kodak 1D 3.6 detector. The intensities of the bands for both isoforms of PRLR and actin were identified according to the molecular weight markers, and the values reported as relative expression of PRLR to actin.
Data Analysis
Data were initially analyzed by a two-way ANOVA using two sets of groups for analysis. Comparisons were first made within reproductive experience groupings (e.g., N-Di, P-D3, and P-D10) and then across lactational states independent of the diestrous groups (P-D3, P-D10, M-D3, and M-D10). Because the gene expression data from the mammary tissue were not normally distributed, the data were transformed to a log scale. Transformed data from the mammary tissue and normally distributed, nontransformed data from liver samples were analyzed for overall group effects and subsequently by post hoc t-tests or Fisher least significant difference tests. The data comparing protein density ratios were analyzed using nonparametric tests, including the Kruskal-Wallis and Mann-Whitney U tests, because the equal variance test failed for these comparisons. An α value of ≤0.05 was set as the criterion for statistical significance for all the tests.
RESULTS
Effects of Reproductive Experience on Prolactin Receptor and Casein Beta Expression and Content in Mammary Tissue
Prlr expression changed both as a function of lactation and reproductive experience (P < 0.05). As shown in Figure 1, in mammary tissue the relative expression of PrlrL was significantly increased on P-D3 (P = 0.002) and P-D10 (P < 0.001) of lactation in all the females when compared to diestrous controls. In experienced females, PrlrL expression was significantly higher in the M-D10 than in P-Di controls (P = 0.01). In addition, the level of expression of the PrlrL in mammary tissue was significantly higher in primiparous rats sampled on D3 and D10 combined when compared with the level of expression present at the comparable times in multiparous dams (P = 0.05). In cycling diestrous females, basal levels of expression for the PrlrL in females with prior reproductive experience, that is, a pregnancy and lactation, although low, were statistically higher than that detected in their age-matched, virgin controls (P = 0.015).
FIG. 1.
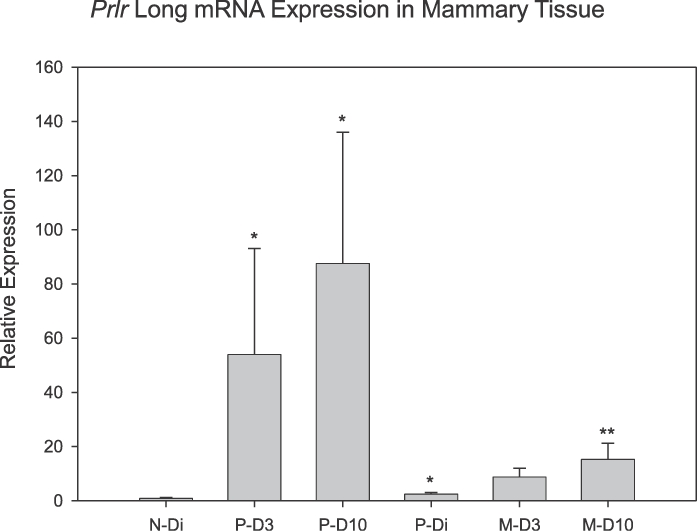
Mean (± SEM) mRNA expression for PrlrL in rat mammary tissue as a function of reproductive state and experience. N-Di, nulliparous, diestrous group; P-D3, primiparous, Day 3 lactating group; P-D10, primiparous, Day 10 lactating group; P-Di, primiparous, nonlactating, diestrous group; M-D3, multiparous, Day 3 lactating group; M-D10, multiparous, Day 10 lactating group. *Denotes significant difference versus the N-Di group (P < 0.05). **Denotes significant difference versus the P-Di group (P = 0.01); n = 5–8/group.
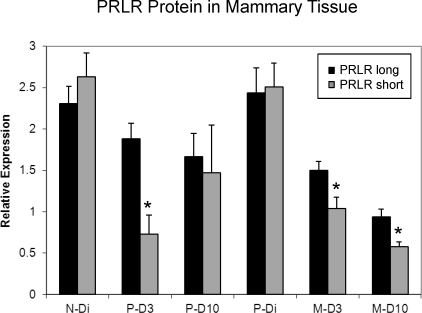
No overall differences were detected in the expression of the PrlrS in mammary tissue (Fig. 2). As with the PrlrL, on both D3 and D10, primiparous lactating rats tended to have increased levels of PrlrS expression when compared with virgin, diestrous controls. These differences did not reach statistical significance. It is noted that as with the expression of PrlrL, the relative expression of the PrlrS declined between the first and second lactation in these age-matched groups (P = 0.029). Comparisons between levels of expression of the PrlrL and PrlrS isoforms revealed that PrlrL was expressed significantly more than PrlrS in the P-D10 (P = 0.015), M-D3 (P = 0.008), and M-D10 (P = 0.003) groups.
FIG. 2.
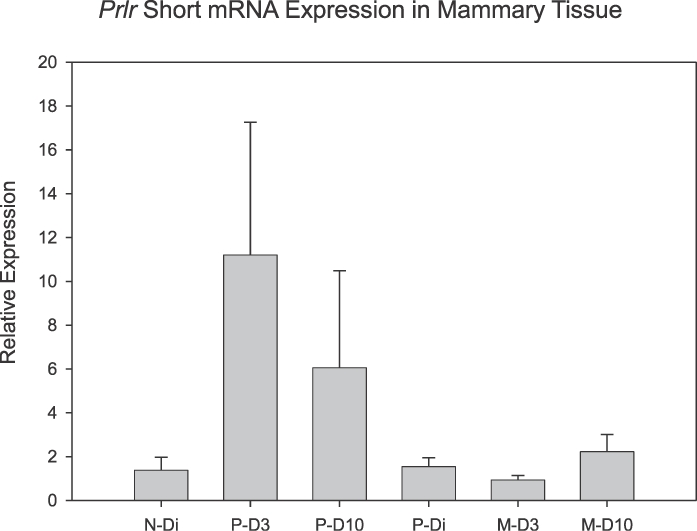
Mean (± SEM) mRNA expression for PrlrS in rat mammary tissue as a function of reproductive state and experience. N-Di, nulliparous, diestrous group; P-D3, primiparous, Day 3 lactating group; P-D10, primiparous, Day 10 lactating group; P-Di, primiparous, nonlactating, diestrous group; M-D3, multiparous, Day 3 lactating group; M-D10, multiparous, Day 10 lactating group; n = 5–8/group.
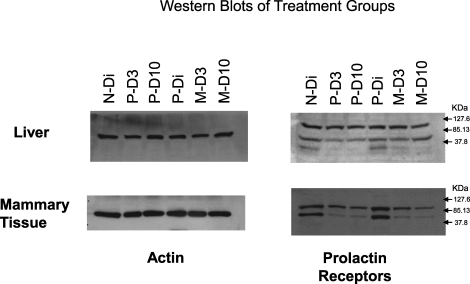
Western blot analysis of PRLRL and PRLRS is shown in Figure 3. Analysis of PRLR protein expression relative to ACTB indicates that the densities of PRLRL are generally higher than those for PRLRS in all but the N-Di group (P < 0.05). Moreover, the ratio of PRLRL:PRLRS was higher during lactation (average ratio = 1.6) than in the diestrous groups (ratios < 1.0). An analysis of the PRLRL:PRLRS ratio among groups demonstrated an overall group effect (P = 0.029). A subsequent comparison between nonlactating diestrous and lactating subjects found that the PRLR protein ratios were significantly higher in the lactating animals (P = 0.001). The ratios of receptor protein densities were comparable in both diestrous groups as they were among the lactating groups. The Western blot depicted in Figure 4 demonstrates reduced densities for the PRLRS isoforms on D3 of lactation in the primiparous animals and on both lactation days in the multiparous groups when compared with the diestrous groups. ACTB band densities were similar across all the treatment groups (Fig. 4).
FIG. 3.
Relative protein expression of the PRLRL and PRLRS in mammary tissue. Values are expressed as means (± SEM). *Denotes significant difference versus PRLRL (P < 0.05); n = 4/treatment group.
FIG. 4.
Western blots of mammary and liver tissue pools (pools of 4–6 animals/treatment group) for PRLRs and actin. Two distinct bands are present in mammary samples processed for the PRLRs. The larger band that is slightly greater than 85.13 kDa is consistent with PRLRL, while the bands between 85.13 and 37.8 kDa are consistent with the molecular weight of the PRLRS isoform. A third faint band of less than 37.8 kDa is also present in the diestrous groups of the hepatic pools.
Casein beta mRNA expression levels in mammary tissues are shown in Figure 5. There was an overall effect of reproductive state on casein beta expression (P < 0.001). Relative casein beta expression levels were significantly higher during lactation than in either nonlactating diestrous group (P = 0.003). On both D3 and D10 of lactation, casein beta expression was markedly elevated in the primiparous dams versus virgin controls (P < 0.01). Comparable elevations in casein beta expression were present in rats undergoing their second lactation (i.e., the multiparous rats) versus diestrous primiparous controls (P = 0.002). Comparisons of casein beta expression between age-matched primiparous and multiparous mothers revealed similar levels of expression as a function of both day of lactation and amount of reproductive experience. Finally, expression of mRNA for casein beta was statistically higher in the age-matched, primiparous diestrous rats when compared with those in the nulliparous diestrous rats (P = 0.016), reflecting an effect of prior reproductive experience on casein beta expression in mammary tissue.
FIG. 5.
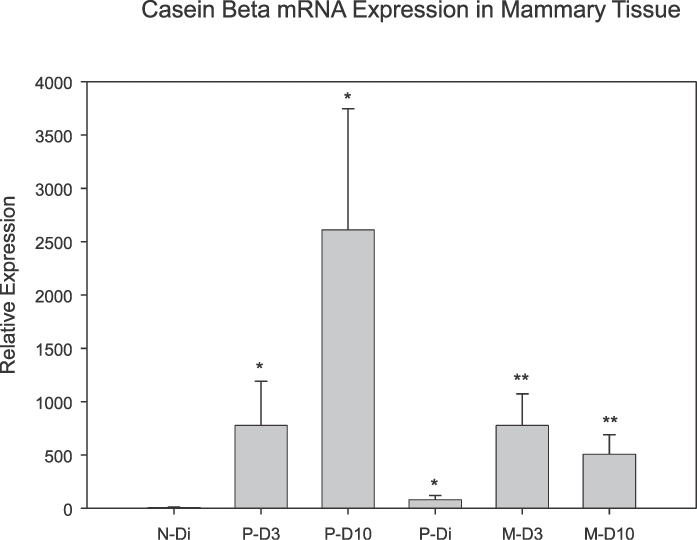
Mean (± SEM) casein beta mRNA expression in mammary tissue samples as a function of reproductive state and experience. *Denotes significant difference versus N-Di group (P < 0.05). **Denotes significant difference versus P-Di group (P < 0.05); n = 5–8/group.
Effects of Reproductive Experience on Prolactin Receptor Expression and Content in Liver
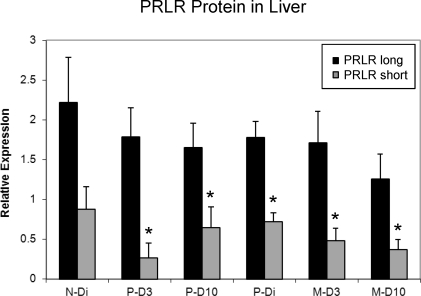
Prlr expression in liver changed as a function of reproductive experience. As shown in Figure 6, expression for PrlrL mRNA was lower during a first lactation when compared to levels of expression during a second lactation (P = 0.024). No differences in PrlrL gene expression were found as a function of day of lactation with levels comparable on D3 and D10 in both reproductive experience groups. Likewise, no day of lactation by reproductive experience interactions were found. As shown in Figure 7, the initial lactation was also associated with significantly lower levels of PrlrS expression (P = 0.039), which was reduced on both D3 (P = 0.027) and D10 (P = 0.035) when compared with diestrous virgin rats. PrlrS mRNA expression was similar in the multiparous lactating groups and their primiparous diestrous controls. Thus, the primary shift in Prlr expression in the liver involved a significant reduction in PrlrS and decreased PrlrL mRNA expression during an initial lactation. Comparisons of expression levels of long versus short isoforms of the Prlr in liver indicate that lactation is associated with increased ratios of PrlrL:PrlrS. When comparing Prlr expressions (Figs. 6 and 7), this ratio increased from about 1:1 in the diestrous groups to approximately 2:1 during lactation.
FIG. 6.
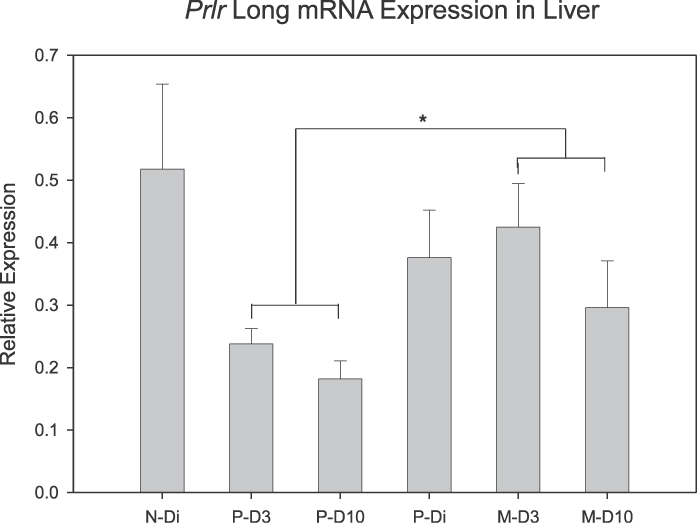
Mean (± SEM) mRNA expression for the PrlrL in hepatic tissue as a function of reproductive state and experience. *Denotes significant difference between P-D3 plus P-D10 versus M-D3 plus M-D10 (P < 0.05); n = 5–8/group.
FIG. 7.
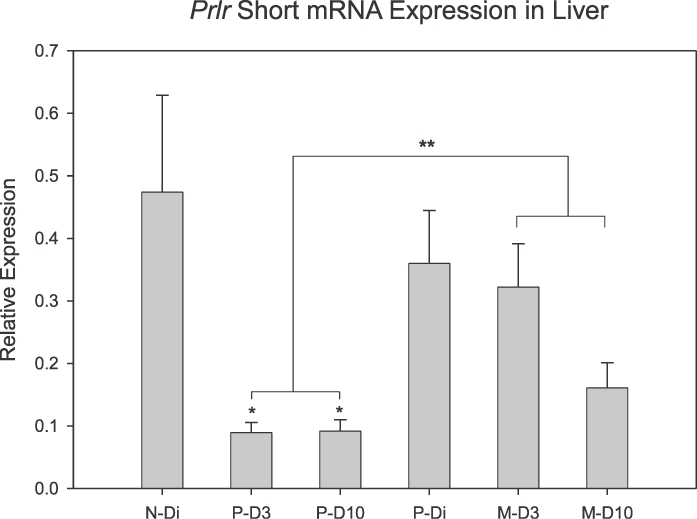
Mean (± SEM) mRNA expression for the PrlrS in hepatic tissue as a function of reproductive state and experience. *Denotes significant difference versus N-Di (P < 0.05). **Denotes significant difference between P-D3 plus P-D10 versus M-D3 plus M-D10 (P < 0.05); n = 5–8/group.
An analysis of Western blot densities in liver for the long and short forms of the PRLR for treatments is shown in Figure 8. The PRLRL concentrations were consistently higher than those of the PRLRS form. Examination of the ratios of hepatic PRLRL:PRLRS revealed that the two diestrous groups had similar ratios of about 2.6, whereas these ratios were higher in the lactating groups, reflecting shifts in PRLR densities in lactating rats. An analysis comparing group receptor ratios revealed a nonsignificant trend toward a group effect (P = 0.108). A subsequent comparison between the diestrous and lactating groups found higher ratios of PRLRL:PRLRS in the lactating groups (P = 0.011). The average ratio of PRLRL:PRLRS for nonlactating diestrous subjects was 2.6, whereas for lactating subjects, the ratio was 4.0. Examination of the Western blots (Fig. 4) indicates that these shifts appear to be associated with reductions in the densities of PRLRS as a function of lactation. No differences were found in hepatic PRLR densities as a function of reproductive experience, that is, primiparous versus multiparous lactating states. Densities of the ACTB bands were similar for all the treatment groups.
FIG. 8.
Relative protein expression of the PRLRL and PRLRS in hepatic tissue. Values are expressed as means (± SEM). *Denotes significant difference versus PRLRL (P < 0.05); n = 4/treatment group.
DISCUSSION
The results of the present study demonstrate that both the states of lactation and prior reproductive experience alter Prlr gene expression in mammary and hepatic tissues. Receptor expression patterns in both mammary and hepatic tissues shifted as a function of reproductive state.
Comparisons of the level of Prlr expression in mammary tissue indicate that during an initial lactation the long form of the receptor is increased and expressed at a higher level than during a second lactation in age-matched rats. Likewise, gene expression of the short form of the receptor is higher in primiparous, lactating rats compared with those of the multiparous mothers. Nevertheless, the ratio of PrlrL:PrlrS expression remained fairly constant on D3 and D10 of lactation in both the primiparous and multiparous groups. Comparison of the ratio of long to short form of the Prlr mRNA among the groups revealed that this ratio was higher in the lactating groups. Whereas the ratios of PrlrL:PrlrS were approximately 1:1 in both tissues in the virgin diestrus and nonlactating, primiparous, diestrous groups, this ratio increased during lactation to a range from 3:1 to 5:1 in mammary tissue and 1.8:1 to 2.7:1 in hepatic tissue. These changes in the ratios of Prlr gene expression may reflect an increase in PRL activity; it has been proposed that PRLRS can act as a dominant negative and thereby reduce PRL signal transduction activity at the receptor level [21, 22]. Thus, a decline in PRLRS availability would be expected to potentiate the actions of the PRLRL and its associated biological actions.
It was unexpected to find that receptor protein product in mammary tissue in general did not parallel that of Prlr gene expression. For example, in mammary tissue the band densities for both forms of the PRLR in the lactating groups were generally lower than in the diestrous groups. In contrast, Prlr gene expression was elevated during lactation compared with diestrous animals. Further studies are warranted to identify the mechanisms involved in this differential Prlr gene expression and protein content.
Casein beta expression, which is, as expected, under PRL regulation [19, 23], was increased during lactation. During lactation, casein beta constitutes a major portion of milk protein. Casein beta is assembled into micelles within milk, which help provide some of the milk's physical characteristics, including stabilizing the milk, reducing the viscosity of the milk, and being able to carry large amounts of calcium phosphate in suspension. Although the levels of casein beta gene expression did not differ between the primiparous and multiparous groups on D3 or D10, there was a slight, yet significant, higher level of casein beta gene expression in the diestrous rats that had previously given birth and raised a litter. Thus, prior reproductive experience appears to affect basal levels of casein beta gene expression. This reproductive experience effect is similar to that reported in neural tissue for PrlrL, that is, nonlactating, cycling rats that have previously raised a litter were found to have higher levels of Prlr expression in both the medial preoptic area and the arcuate region compared to levels present in age-matched virgin rats [10]. Further work is needed to assess the effects of reproductive experience on casein beta protein content because measurement of mRNA expression provides only one perspective of the effects of reproductive experience on casein beta's biological activity.
In the liver, gene expression of both forms of the Prlr was lower during an initial lactation when compared with levels of expression in both the diestrous groups and the multiparous subjects. Messenger RNA expression levels for both forms of Prlr were comparable between the diestrous groups and the multiparous animals on both D3 and D10 of lactation. The most pronounced change in hepatic Prlr gene expression was present in PrlrS mRNA expression in the primiparous groups on D3 and D10 of lactation when it declined. Those factors responsible for the decline in hepatic mRNA expression for PrlrS or the consequences of these declines are unknown. However, such changes may affect bile production/transport and lipid metabolism differentially during an initial lactation.
What is the overall impact of reproductive experience on PRLR systems? When the results of the present study are taken together with prior studies that focused on the neural PRLR system, some overall patterns are found. Notably, the Prlr expression levels change as a function of the number of pregnancies and lactations in multiple PRL target tissues. In both brain and liver, there is an increase in PrlrL gene expression with increasing amounts of reproductive experience. In mammary tissue, in contrast, expression of both PrlrS and PrlrL decline between the initial and second lactation. The physiological significance of these changes is not clearly understood. However, given the higher level of PRL-stimulated milk production associated with a second lactation [1, 24], it is possible that these changes in mammary Prlr expression and receptor protein may participate in the elevations in lactogenesis in multiparous animals. It is possible that the changes in receptor isoform protein ratios found in this study may underlie a shift in sensitivity of the mammary glands with the PRLRS acting as a dominant negative. In mice, both pregnancy and prior parity render the mammary glands more responsive to hormonal induction of lactose synthetase activity [16]. Related studies in rats have reported that parity results in increased estrogen receptor expression in the mammary gland with increased expression of both ESR1 (previously ER alpha) and ESR2 (previously ER beta) detected in multiparous dams [17]. In humans, milk intake in neonates of multiparous mothers is greater than that in neonates of primiparous mothers [24]. Moreover, body weight gains of the neonates of multiparous mothers are higher, presumably reflecting some combination of greater PRLR occupation and activity during the first few days of life and/or enhanced milk letdown and altered nursing patterns. Multiple lactations also enhance lactogenesis in mice, that is, in mice heterozygous for the Prlr gene, lactational performance is poor following an initial birth but is capable of sustaining the growth of young after a second birth [25, 26]. Thus, it is apparent that the ability of mammary tissue to respond to the endocrine and sensory signals during a second lactation may be enhanced compared with that present in primiparous mothers.
The shift in PRLR expression in hepatic tissue between the first and second lactation may functionally affect bile transport. Prolactin increases maternal bile secretion in postpartum as compared with pregnant or nonpregnant controls [15]. Moreover, increased expression of the bile salt transporters Slc10a1 (formerly Ntcp) and Abcb11 (formerly Bsep) and proteins have been reported to occur in postpartum lactating rats, an increase potentiated by PRL treatment [27]. Prolactin also increases ATP-dependent taurocholate transport in canalicular plasma membrane from rat liver that is associated with increased bile transport [28]. It has been suggested that the increased bile secretion and the activity of these transporters may function to meet the maternal nutritional needs to increase absorption of fats and fat-soluble vitamins [27]. An increase in hepatic function in response to elevated lipid levels may help modulate excessive fat content in the maternal circulation.
That multiple pregnancies resulted in a higher level of PrlrL mRNA expression in hepatic tissue parallels the effects of a prior parity on the expression of PrlrL in brain [10]. A similar increase in PrlrS expression was found in hepatic tissues. The physiological consequences of these shifts are unknown given that these changes in gene expression were not associated with comparable changes in PRLR protein in liver. This was surprising but may reflect differences in posttranslational processing of the receptors or alterations in degradation rates. Future studies that examine these shifts are warranted in order to gain a better understanding of the dynamics between receptor gene expression, receptor protein content, and the biological significance of these changes in the PRLR systems.
It is noted that measurements of Prlr gene expression in the present study did not examine the possible intracellular compartmentalization of PRLRs within either mammary or hepatic tissues. Posner et al.'s [29] study of hepatic PRLR localization in rats identified receptors both on the cell surface membrane and internalized in the Golgi. Moreover, during lactation a higher percentage of PRLRs were localized in the Golgi. These authors proposed that the location of the PRLR sites may affect the actions of ligand bound to the receptor in the liver. Similar studies have identified both an acid-sensitive and acid-insensitive subset of PRLRs in mammary tissue [30]. Specifically, during lactation the level of acid-sensitive receptors increase more slowly than that of acid-insensitive receptors located primarily on intracellular membranes. Likewise, it is noted that PRLRs are expressed in both mammary epithelial cells and stroma with increases in the long form of the receptor present in the epithelial compartment during lactation [31, 32]. It is possible that the differences in expression of receptors in the mammary gland in the present study result from changes in the relative number of epithelial cells associated with the various reproductive states examined. Thus, whereas our determinations of PRLR subtype content in these tissues revealed alterations in the isoforms of the PRLR during lactation, future studies are needed to examine the precise compartmentalization of these receptors.
The regulation of PRLR expression likely reflects a time-dependent, ligand-receptor interaction. It is well established that PRL stimulates PRLR concentrations in mammary [33], hepatic [34, 35] and neural [36] tissues. The stimulatory effects of PRL on receptor numbers in these tissues likely are accompanied by temporally dependent up-regulation [10, 11, 33–35] and down-regulation [37] in PRLR protein and gene expression. Thus, assessments of ligand concentrations, receptor numbers, occupation, and turnover as well as receptor gene expression are important determinants in clarifying the biological impact of shifts and levels in Prlr gene expression.
Finally, that reproductive experience reduces circulating PRL levels [7–9] and alters Prlr expression in mammary tissue is of interest in light of the possible role of PRL in breast cancer [38, 39] and the protective effects of giving birth when a woman is in her twenties upon the subsequent incidence of breast cancer [40, 41]. Whether reproductive experience in women induces similar shifts in the sensitivity of PRLRs and PRL actions in mammary tissue that participate in protecting the female from the development of breast cancer is not known. It would be of interest to determine if a shift in PRL activity occurs in women that may play a role in the development of breast cancer.
In summary, the present study demonstrates that multiple reproductive experiences modify Prlr gene expression and receptor protein densities at two key sites of PRL action, the mammary gland and liver. The precise physiological consequences of these changes likely include improved milk production in multiparous mothers as well as alterations in fat metabolism. These findings together with earlier ones that demonstrate shifts in receptor expression and activation of PRL transduction pathways in neural tissues as a consequence of reproductive experience [10, 11] emphasize the importance of weighing the impact of single and multiple reproductive experiences on a range of endocrine functions related to the health status of the female across her lifespan.
ACKNOWLEDGMENT
We thank Dennis Lovelock for his assistance in the preparation of the figures.
Footnotes
Supported by National Institutes of Health grant R01 HD39895 (R.S.B.).
REFERENCES
- Forsyth IA. Variation among species in the endocrine control of mammary growth and function: the roles of prolactin, growth hormone, and placental lactogen. J Dairy Sci 1986; 69: 886 903. [DOI] [PubMed] [Google Scholar]
- Woodside B, Augustine RA, Ladyman SR, Naef L, Grattan DR. Role of prolactin in the metabolic adaptations to pregnancy and lactation. : Bridges RS. (ed.), Neurobiology of the Parental Brain. San Diego: Academic Press; 2008: 249 268. [Google Scholar]
- Grattan DR. The actions of prolactin in the brain during pregnancy and lactation. Prog Brain Res 2001; 133: 153 171. [DOI] [PubMed] [Google Scholar]
- Torner L, Toschi N, Nava G, Clapp C, Neumann ID. Increased hypothalamic expression of prolactin in lactation: involvement in behavioural and neuroendocrine stress responses. Eur J Neurosci 2002; 15: 1381 1389. [DOI] [PubMed] [Google Scholar]
- Bridges RS, DiBiase R, Loundes DD, Doherty PC. Prolactin stimulation of maternal behavior in female rats. Science 1985; 27: 782 784. [DOI] [PubMed] [Google Scholar]
- Tucker HA. Endocrinology of lactation. Semin Perinatol 1979; 3: 199 233. [PubMed] [Google Scholar]
- Musey VC, Collins DC, Musey PI, Martino-Saltzman D, Preedy JRK. Long-term effect of a first pregnancy on the secretion of prolactin. New Engl J Med 1987; 316: 229 234. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Byrnes EM. Reproductive experience reduces circulating estradiol-17β and prolactin levels during proestrus and alters estrogen sensitivity in female rats. Endocrinology 2006; 147: 2575 2582. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Henriquez BM, Sturgis JD, Mann PE. Reproductive experience reduces haloperidol-induced prolactin secretion in female rats. Neuroendocrinology 1997; 66: 321 326. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Grattan DR, van den Ancker W, Bridges RS. Reproductive experience increases prolactin responsiveness in the medial preoptic area and arcuate nucleus of female rats. Endocrinology 2006; 147: 4688 4694. [DOI] [PubMed] [Google Scholar]
- Sjoeholm A, Bridges RS, Grattan DR, Anderson GM. Region, neuron and signaling pathway-specific increases in prolactin responsiveness in reproductively experienced female rats. Endocrinology 2011; 152: 1978 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes SR, Rogers RL, Naylor MJ, Ormandy CJ. Prolactin regulation of mammary gland development. J Mammary Gland Biol Neoplasia 2008; 13: 13 28. [DOI] [PubMed] [Google Scholar]
- Bogorad RI, Ostroukhova TY, Orlova AN, Rubtsov PM. Long isoforms of prolactin receptor predominates in rat intrahepatic bile ducts and further increases under obstructive cholestasis. J Endocrinol 2006; 188: 345 354. [DOI] [PubMed] [Google Scholar]
- Varas SM, Jahn GA. The expression of estrogen, prolactin, and progesterone receptors in mammary gland and liver of female rats during pregnancy and early postpartum: regulation by thyroid hormones. Endocr Res 2005; 31: 357 370. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hyde J, Vore M. Prolactin regulates maternal bile secretory function postpartum. J Pharmacol Expr Ther 1992; 261: 560 566. [PubMed] [Google Scholar]
- Bolander FF. Persistent alterations in hormonal sensitivities of mammary glands from parous mice. Endocrinology 1983; 112: 1796 1800. [DOI] [PubMed] [Google Scholar]
- Kass L, Durando M, Ramos JG, Varayoud J, Powell CE, Luque EH, Munoz-de-Toro M. Association of increased estrogen receptor β2 expression with parity-induced alterations in the rat mammary gland. Steroid Biochem Mol Biol 2004; 91: 29 39. [DOI] [PubMed] [Google Scholar]
- Okamura H, Zachwieja J, Raguet S, Kelly PA. Characterization and applications of monoclonal antibodies to the prolactin receptor. Endocrinology 1989; 124: 2499 2508. [DOI] [PubMed] [Google Scholar]
- Lee CS, Oka T. Progesterone regulation of a pregnancy-specific transcription repressor of β-casein gene promoter in mouse mammary gland. Endocrinology 1992; 131: 2257 2262. [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Bridges RS. Reproductive experience and expression of dopamine D2 receptor mRNA: a possible mechanism for reduced prolactin secretion in primiparous rats. J Neuroendocrinol 2007; 19: 773 778. [DOI] [PubMed] [Google Scholar]
- Berlanga JJ, Garcia-Rutz JP, Perrot-Applanat M, Kelly PA, Edery M. The short form of the prolactin (PRL) receptor silences induction of the β-casein gene promoter. Mol Endocrinol 1997; 11: 1449 1457. [DOI] [PubMed] [Google Scholar]
- Perrot-Applanat M, Gualilio O, Pezet A, Vincent V, Edery M, Kelly PA. Dominant negative and cooperative effects of mutant forms of prolactin receptor. Mol Endocrinol 1997; 11: 1020 1032. [DOI] [PubMed] [Google Scholar]
- Guyette WA, Matusik RJ, Rosen JM. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell 1979; 17: 1013 1023. [DOI] [PubMed] [Google Scholar]
- Zuppa AA, Tornesell A, Papacci P, Tortorolo G, Segni G, Lafuenti G, Moneta E, Diodato A, Sorcini M, Carta S. Relationship between maternal parity, basal prolactin levels and neonatal breast milk intake. Biol Neonate 1988; 53: 144 147. [DOI] [PubMed] [Google Scholar]
- Ormandy C, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin gene produces multiple reproductive defects in the mouse. Genes Dev 1997; 11: 167 178. [DOI] [PubMed] [Google Scholar]
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocrinol Rev 1998; 19: 225 268. [DOI] [PubMed] [Google Scholar]
- Cao J, Huang L, Lui Y, Hoffman T, Stieger B, Meier PJ, Vore M. Differential regulation of hepatic bile salt and organic anion transporters in pregnant and postpartum rats and the role of prolactin. Hepatology 2001; 33: 140 147. [DOI] [PubMed] [Google Scholar]
- Liu Y, Suchy FL, Silverman JA, Vore M. Prolactin increases ATP-dependent taurocholate transport in canalicular plasma membrane from rat liver. Am J Physiol 1997; 272: G46 G53. [DOI] [PubMed] [Google Scholar]
- Posner BI, Josefsberg Z, Bergerson JJM. Intracellular polypeptide hormone receptors. J Biol Chem 1979; 254: 12494 12499. [PubMed] [Google Scholar]
- Sakai S, Mizoguchi Y, Kim JY. Characterization of plasma and intracellular membrane prolactin receptor in lactating mouse mammary cells. Endocr J 1994; 41: 249 256. [DOI] [PubMed] [Google Scholar]
- Camarillo IG, Thordarson G, Moffat JG, Van Horn KM, Binart N, Kelly PA, Talamantes F. Prolactin receptor expression in the epithelia and stroma of the rat mammary gland. J Endocrinol 2001; 171: 85 95. [DOI] [PubMed] [Google Scholar]
- Hovey RC, Trott JF, Ginsburg E, Goldhar A, Sasaki MM, Fountain SJ, Sundararajan K, Vonderhaar BK. Transcriptional and spatiotemporal regulation of prolactin receptor mRNA and cooperativity with progesterone receptor function during ductal branch growth in the mammary gland. Dev Dyn 2001; 222: 192 205. [DOI] [PubMed] [Google Scholar]
- Djiane J, Durand P. Prolactin-progesterone antagonism in self regulation of prolactin receptors in the mammary gland. Nature 1977; 266: 641 643. [DOI] [PubMed] [Google Scholar]
- Posner BI, Kelly PA, Friesen HG. Induction of lactogenic receptor in rat liver: influence of estrogen and the pituitary. Proc Natl Acad Sci U S A 1974; 71: 2407 2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash T, Cromlish W, Posner BI. Prolactin (PRL) receptor localization in cultured rat hepatocytes: dual regulation by PRL and growth hormone. Endocrinology 1988; 122: 1151 1158. [DOI] [PubMed] [Google Scholar]
- Muccoli G, DiCarlo R. Modulation of prolactin receptors in the rat hypothalamus in response to changes in serum concentrations of endogenous prolactin or to ovine prolactin administration. Brain Res 1994; 663: 244 250. [DOI] [PubMed] [Google Scholar]
- Djiane J, Clauser H, Kelly PA. Rapid down-regulation of prolactin receptors. Biochem Biophys Res Com 1979; 90: 1371 1378. [DOI] [PubMed] [Google Scholar]
- Ewertz M, Duffy SW. Risk of breast cancer in relation to reproductive factors in Denmark. Br J Cancer 1988; 58: 99 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe AM, Willett WC, Xue F, Michels KB. Lactation and incidence of premenopausal breast cancer. Arch Intern Med 2009; 169: 1364 1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampert JB, Whittemore AS, Paffenbarger RS. Combined effect of childbearing, menstrual events, and body size on age-specific breast cancer risk. Am J Epidemiol 1988; 128: 962 979. [DOI] [PubMed] [Google Scholar]
- Leon DA. A prospective study of the independent effect of parity and age at first birth on breast cancer incidence in England and Wales. Int J Cancer 1989; 43: 986 991. [DOI] [PubMed] [Google Scholar]