Abstract
Shoot elongation is a vital process for plant development and productivity, in both ecological and economic contexts. Auxin and bioactive gibberellins (GAs), such as GA1, play critical roles in the control of elongation,1–3 along with environmental and endogenous factors, including other hormones such as the brassinosteroids.4,5 The effect of auxins, such as indole-3-acetic acid (IAA), is at least in part mediated by its effect on GA metabolism,6 since auxin upregulates biosynthesis genes such as GA 3-oxidase and GA 20-oxidase and downregulates GA catabolism genes such as GA 2-oxidases, leading to elevated levels of bioactive GA1.7 In our recent paper,1 we have provided evidence that this action of IAA is largely independent of DELLA proteins, the negative regulators of GA action,8,9 since the auxin effects are still present in the DELLA-deficient la cry-s genotype of pea. This was a crucial issue to resolve, since like auxin, the DELLAs also promote GA1 synthesis and inhibit its deactivation. DELLAs are deactivated by GA, and thereby mediate a feedback system by which bioactive GA regulates its own level.10 However, our recent results,1 in themselves, do not show the generality of the auxin-GA relationship across species and phylogenetic groups or across different tissue types and responses. Further, they do not touch on the ecological benefits of the auxin-GA interaction. These issues are discussed below as well as the need for the development of suitable experimental systems to allow this process to be examined.
Key words: auxin, gibberellins, DELLA proteins, interactions, elongation
Generality of the Auxin-GA Interaction
The strong promotion of bioactive GA levels by auxin appears to occur widely across angiosperms. It is present in both monocots (barley11) and broadly across the dicots (pea,12 Arabidopsis1,13 and tobacco14). However, the molecular basis of the interaction may differ from species to species. For example, with respect to GA1 synthesis, the shoot-expressed GA20ox1 gene seems to be only marginally upregulated in pea shoots, with the main effect attributable to the upregulation of Mendel's GA 3-oxidase gene, LE.12,15 However, in tobacco GA 20-oxidation is strongly upregulated.14 The reason for this difference is not clear, although at least two hypotheses can be suggested. Firstly, since the two gene families are closely related members of the 2-oxoglutarate-dependent dioxygenase group of enzymes, the auxin-GA interaction might pre-date the evolution of the split into these two enzyme specificities. Secondly, this may be a case of convergent evolution to yield the required effect on GA1 levels because of the evolutionary importance to regulate elongation.
Developmental Responses
While the work by O'Neill et al.1 focused on shoot elongation in pea and Arabidopsis, the effect of auxin on stimulating GA1 levels is also seen in pea roots,15 pods and seeds.16,17 It therefore appears to be a common regulatory junction between the auxin and GA pathways. Detailed examination of these interactions in DELLA-deficient la cry-s plants has not been carried out for all these developmental processes, so it is not clear if the action of auxin is independent of DELLA action. However, in roots the auxin action inhibitor PCIB caused significantly less inhibition of elongation in la cry-s plants than in phenotypically wild type plants, although this does not distinguish between the effect of auxin on GA synthesis or an effect on the GA response pathway.15 Likewise, decapitation did not reduce the elongation of young expanding internodes in the DELLA-deficient pea mutant la cry-s (at this stage of development) as it does in plants with a wild-type phenotype (Table 1, p < 0.01). This presumably occurs since GA1 levels and hence auxin levels are not important for elongation in the absence of the growth-inhibiting DELLA proteins. In pea pods (pericarps) it appears that it is only the chlorinated auxin, 4-Cl-IAA, and not IAA, that is involved in the regulation of GA metabolism genes.17 This is suggested to be due to the ability of 4-Cl-IAA, but not IAA, to inhibit ethylene action.18 The 4-Cl-IAA is thought to be exported from the developing seed to ensure pod growth is sufficient to allow seed development.17 In la cry-s plants parthenocarpic pods of normal size develop from emasculated flowers showing that DELLA proteins are involved in pod development.19
Table 1.
Elongation of the upper internode of phenotypically wild-type and the DE LLA-deficient la cry-s mutant of pea seedlings either left intact or decapitated directly below the apical bud
| Intact | Decapitated | |||
| (cm) | n | (cm) | n | |
| Wild-type | 1.82 ± 0.13 | 6 | 1.29 ± 0.07 | 6 |
| la cry-s | 3.25 ± 0.46 | 4 | 3.63 ± 0.54 | 3 |
Elongation was measured over the 48 h period after decapitation. The plants were 12 days old at the time of decapitation.
A problem with studying plant hormones is how to perturb the system in a biologically relevant way. This is particularly the case where a hormone shows a clear optimum level for the control of a process, as is evident for auxin in stem and root elongation.1,20 It is well known that auxin at high concentrations can inhibit elongation, but such concentrations, and the resulting internal levels of the applied compound, are probably unrealistically high and never encountered naturally. We suggest it is preferable to deplete auxin using auxin transport inhibitors,21 decapitation12,22 or excised segments.1 This allows strong responses to be observed, both to the depletion and also to the addition of auxin to the auxin-depleted system. This may be much more important for work on auxin than for other hormones (e.g., GA, brassinosteroids) where distinct optima do not appear to exist and only saturation of the response is observed at high physiological concentrations.4,23 The cause of this difference between auxin and other hormones is beyond the scope of this article but may reflect the direct effects that auxin has on other hormone levels, especially ethylene.24 A further difficulty in working with auxin is that unlike other hormones, clear IAA-deficient mutants, specifically impaired in IAA biosynthesis, are unavailable.
Ecological Implications
Removal of the shoot apex has a major impact on the competitive capability of plants. This is particularly the case in herbaceous, mesophyllic, caulescent dicots where the apical bud is exposed (terminal) and the plants are competing for space and light. However, after removal of the apex, apical dominance is released, allowing the shoot to regenerate. As part of this response the plant must redirect its resources from the elongating shoot that has been decapitated to the new laterals that have been released from dormancy or growth suppression. In pea, for example, the decapitated internode remaining can be less than 10% of the fresh weight of the fully expanded internode (data not shown). Traditionally, the drop in basipetal auxin transport from the shoot apex has been implicated in the outgrowth of lateral buds, although this involvement has been questioned.25
Interactions between auxin and other signals, including the recently identified branching hormone, strigolactone, are still being clarified.26 The drop in GA1 levels in the decapitated stem, attributable to reduced auxin content, is a vital component in this redistribution of resources, reducing elongation of the “stump” and internodes below this that are still elongating (Wolbang and Ross, unpub. data), thereby freeing up nutrition that presumably can be diverted into the new lateral shoot(s) (Fig. 1).
Figure 1.
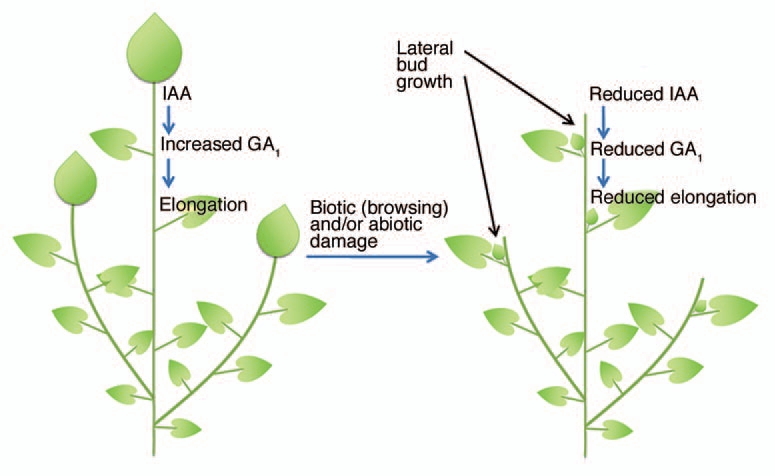
Biotic and abiotic removal of shoot apices results in reduced stem growth and conservation of resources for lateral bud outgrowth. This occurs since apex removal reduces auxin levels in young internodes which results in reduced bioactive GA levels and hence elongation.
Experimental Approaches
With the increasing ease of monitoring gene expression, it has become common to base the suggested involvement of a hormone (i.e., the level of the hormone) in a particular process on changes in the expression of hormone synthesis/metabolism genes, rather than measuring the level of the hormone directly.13,27,28 The validity of this approach has been discussed in the literature.29 A major problem with using the expression of metabolism genes is that most are members of multi-gene families. Further, the pathways involved have many steps. Hence, it is hard to infer, even from comprehensive studies, what the level of the biologically active member of the pathway may be. Direct physiochemical measurements are frequently the only way to be certain about the hormone level, although such methods suffer from the need for relatively large tissue samples (at least mg quantities) and therefore may not provide a measure in a certain cell type or even tissue type. Molecular techniques may in future provide this specificity although the risk of other factors impinging on the reporter system needs to be considered, possibly on a case-by-case basis.
Even where the level of the active hormone is accurately measured it may still be important to use metabolic studies with labelled intermediates to understand why this level has changed. For example, in the original work on the effect of auxin on GA1 levels12 it was not clear if GA1 levels were changed solely because of enhanced synthesis or by changes to both synthesis and catabolism. With multi-gene families involved with both processes and auxin sometimes regulating members of these families in opposite directions, metabolism studies are the only easy method for summing the actions of the gene family at the metabolite level, as was done in O'Neill et al.1
References
- 1.O'Neill DP, Davidson SE, Clarke VC, Yamauchi Y, Yamaguchi S, Kamiya Y, et al. Regulation of the gibberellin pathway by auxin and DELLA proteins. Planta. 2010;232:1141–1149. doi: 10.1007/s00425-010-1248-0. [DOI] [PubMed] [Google Scholar]
- 2.Davies PJ. The plant hormones: Their nature, occurrence and functions. In: Davies PJ, editor. Plant Hormones: Biosynthesis, Signal Transduction, Action! Dordrecht: Springer; 2010. pp. 1–15. [Google Scholar]
- 3.McKay MJ, Ross JJ, Lawrence NL, Cramp RE, Beveridge CA, Reid JB. Control of internode length in Pisum. Further evidence for the involvement of indole-3-acetic acid. Plant Physiol. 1994;106:1521–1526. doi: 10.1104/pp.106.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nomura T, Nakayama M, Reid JB, Takeuchi Y, Yokota T. Blockage of brassinosteroid biosynthesis and sensitivity causes dwarfism in garden pea. Plant Physiol. 1997;113:31–37. doi: 10.1104/pp.113.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nomura T, Bishop GJ, Kaneta T, Reid JB, Chory J, Yokota T. The LKA gene is a BRASSINOSTEROID INSENSITIVE 1 homolog of pea. Plant J. 2003;36:291–300. doi: 10.1046/j.1365-313x.2003.01863.x. [DOI] [PubMed] [Google Scholar]
- 6.Ross JJ, O'Neill DP, Rathbone DA. Auxin-gibberellin interactions in pea: integrating the old with the new. J Plant Growth Regul. 2003;22:99–108. [Google Scholar]
- 7.O'Neill DP, Ross JJ. Auxin regulation of the gibberellin pathway in pea. Plant Physiol. 2002;130:1974–1982. doi: 10.1104/pp.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun T, Gubler F. Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol. 2004;55:197–223. doi: 10.1146/annurev.arplant.55.031903.141753. [DOI] [PubMed] [Google Scholar]
- 9.Weston DE, Elliott RC, Lester DE, Rameau C, Reid JB, Murfet IC, et al. The pea (Pisum sativum) DELLA proteins LA and CRY are important regulators of gibberellin synthesis and root growth. Plant Physiol. 2008;147:199–205. doi: 10.1104/pp.108.115808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross JJ, MacKenzie-Hose AK, Davies PJ, Lester DR, Twitchin B, Reid JB. Further evidence for feedback regulation of gibberellin biosynthesis in pea. Physiol Plant. 1999;105:532–538. [Google Scholar]
- 11.Wolbang CM, Chandler PM, Smith JJ, Ross JJ. Auxin from the developing inflorescence is required for the biosynthesis of active gibberellins in barley stems. Plant Physiol. 2004;134:769–776. doi: 10.1104/pp.103.030460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross JJ, O'Neill DP, Smith JJ, Kerckhoffs LHJ, Elliott RC. Evidence that auxin promotes gibberellin A1 biosynthesis in pea. Plant J. 2000;21:547–552. doi: 10.1046/j.1365-313x.2000.00702.x. [DOI] [PubMed] [Google Scholar]
- 13.Frigerio M, Alabadí D, Pérez-Gómez J, García-Cárcel L, Phillips AL, Hedden P, et al. Transcriptional regulation of gibberellin metabolism genes by auxin signaling in Arabidopsis. Plant Physiol. 2006;142:553–563. doi: 10.1104/pp.106.084871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolbang CM, Ross JJ. Auxin promotes gibberellin biosynthesis in decapitated tobacco plants. Planta. 2001;214:153–157. doi: 10.1007/s004250100663. [DOI] [PubMed] [Google Scholar]
- 15.Weston DE, Reid JB, Ross JJ. Auxin regulation of gibberellin biosynthesis in the roots of pea (Pisum sativum L.) Funct Plant Biol. 2009;36:362–369. doi: 10.1071/FP08301. [DOI] [PubMed] [Google Scholar]
- 16.Ozga JA, Yu J, Reinecke DM. Pollination-, development- and auxin-specific regulation of gibberellin-3β-hydroxylase gene expression in pea fruit and seeds. Plant Physiol. 2003;131:1137–1146. doi: 10.1104/pp.102.015974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozga JA, Reinecke DM, Aylele BT, Ngo P, Nadeau C, Wickramarathna AD. Developmental and hormonal regulation of gibberellin biosynthesis and catabolism in pea fruit. Plant Physiol. 2009;150:448–462. doi: 10.1104/pp.108.132027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnstone MMG, Reinecke DM, Ozga JA. The auxins IAA and 4-Cl-IAA differentially modify gibberellin action via ethylene response in developing pea fruit. J Plant Growth Regul. 2005;24:214–225. [Google Scholar]
- 19.Ross JJ, Reid JB, Murfet IC, Weston DE. The slender phenotype of pea is deficient in DELLA proteins. Plant Signal Behav. 2008;3:590–592. doi: 10.4161/psb.3.8.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eliasson L, Bertell G, Bolander E. Inhibitory action of auxin on root elongation not mediated by ethylene. Plant Physiol. 1989;91:310–314. doi: 10.1104/pp.91.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross JJ. Effects of auxin transport inhibitors on gibberellins in pea. J Plant Growth Regu. 1998;17:141–146. [Google Scholar]
- 22.Fu X, Harberd NP. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature. 2003;421:740–743. doi: 10.1038/nature01387. [DOI] [PubMed] [Google Scholar]
- 23.Reid JB, Ross JJ. Internode length in Pisum. A new gene, lv, conferring an enhanced response to gibberellin A1. Physiol Plant. 1988;72:595–604. [Google Scholar]
- 24.Abeles FB. Ethylene in plant biology. New York: Academic Press; 1973. [Google Scholar]
- 25.Morris SE, Ross JJ, Cox MCH, Krisantini S, Beveridge CA. Auxin dynamics after decapitation are not correlated with the initial outgrowth of axillary buds. Plant Physiol. 2005;138:1665–1672. doi: 10.1104/pp.104.058743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson BJ, Beveridge CA. Roles of auxin, cytokinin and strigolactone in regulating shoot branching. Plant Physiol. 2009;149:1929–1944. doi: 10.1104/pp.109.135475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang JG, Yun J, Kim DH, Chung KS, Fujioka S, Kim JI, et al. Light and brassinosteroid signals are integrated via a dark-induced small G protein in etiolated seedling growth. Cell. 2001;105:625–636. doi: 10.1016/s0092-8674(01)00370-1. [DOI] [PubMed] [Google Scholar]
- 28.Du L, Poovaiah BW. Ca2+/calmodulin is critical for brassinosteroid biosynthesis and plant growth. Nature. 2005;437:741–745. doi: 10.1038/nature03973. [DOI] [PubMed] [Google Scholar]
- 29.Symons GM, Reid JB. Brassinosteroids, de-etiolation and the re-emerging art of plant hormone quantification. Plant Signal Behav. 2008;3:868–870. doi: 10.4161/psb.3.10.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]


