Table 2. Summary of drugs targeting the PI3K pathway in clinical trials for cancer treatment*.
| Cateogory | Agent | Target | Sponsor | Phase | Cancer Type/Condition | Chemical structure |
|---|---|---|---|---|---|---|
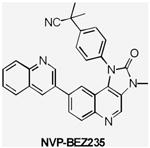
| PI3K Inhibitors | BEZ235 | Class I PI3K/mTOR | Novartis | Phase I/II | Advanced solid tumors Advanced Breast cancer |
|
|
| ||||||
| BGT226 | Class I PI3K/mTOR | Novartis | Phase I/II | Solid tumors Advanced breast cancer Cowden syndrome |
ND | |
|
| ||||||
| BKM120 | Class I PI3K | Novartis | Phase I (1st Qtr 2009) |
Solid tumors | ND | |
|
| ||||||
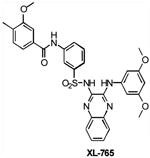
| XL765 | Class I PI3K/mTOR | Exelixis | Phase I | Solid tumors Non-Small Cell Lung Cance Malignant Gliomas |
|
|
|
| ||||||
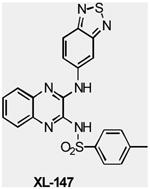
| XL147 | Class I PI3K | Exelixis | Phase I | Advanced solid tumors Endometrial Carcinoma Ovarian Carcinoma Non-Small Cell Lung Cance |
|
|
|
| ||||||
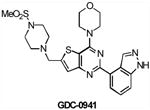
| GDC0941 | Class I PI3K | Genentech | Phase I | Advanced solid tumors Non-Hodgkin's lymphoma |
|
|
|
| ||||||
| SF1126 | pan-PI3K/mTOR | Semafore | Phase 1 | Advanced solid tumors |
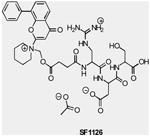
|
|
|
| ||||||
| GSK1059615 | pan-PI3K | GlaxoSmithKline | Phase I | Advanced solid tumors Metastatic breast cancer Endometrial cancer Lymphoma |
ND | |
|
| ||||||
| PX-866 | PI3K (α, δ, and γ) | Oncothyreon | Phase 1 | Advanced solid tumors |
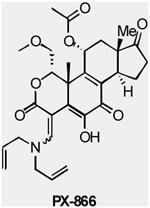
|
|
|
| ||||||
| CAL-101 | PI3K (δ) | Calistoga | Phase I | Chronic lymphocytic leukemia (CLL) Acute myeloid leukemia (AML) Non-Hodgkin's lymphoma |
ND | |
|
| ||||||
| Akt Inhibitors | Perifosine (KRX-0401) |
AKT | Keryx | Phase I/II | Advanced solid tumors Multiple myeloma Ovarian cancer Soft Tissue sarcoma Malignant melanoma |
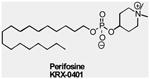
|
|
| ||||||
| MK2206 | AKT | Merck | Phase I | Advanced solid tumors |
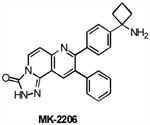
|
|
|
| ||||||
| VQD-002 (API-2/TCN) |
AKT | VioQuest | Phase I | Hematologic malignancies Leukemia Non-small cell lung cancer |
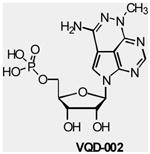
|
|
|
| ||||||
| XL418 | AKT/ S6K | Exelixis | Phase I** | Solid Tumors | ND | |
|
| ||||||
| mTOR Inhibitors | Rapamycin (Sirolimus, Rapamune®) |
mTORC1 | Wyeth | Phase I/II | Advanced solid tumors Metastatic breast cancer Myeloid leukemia |
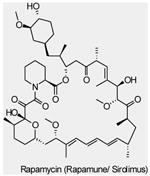
|
|
| ||||||
| Approved | Advanced renal cell carcinoma (RCC) |
|
||||
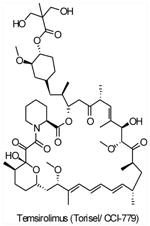
| CCI-779 (Temsirolimus,Torisel®) |
mTORC1 | Wyeth | Phase I/II/III | Advanced solid tumors Multiple myeloma Ovarian cancer Endometrial Cancer Mantle cell lymphoma Brain tumors Non-small cell lung cancer Malignant melanoma |
||
|
| ||||||
| Approved | Advanced renal cell carcinoma (RCC) |
|
||||
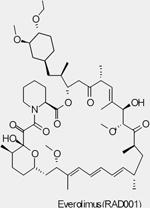
| RAD001 (Everolimus, Afinitor®) |
mTORC1 | Novartis | Phase I/II/III | Advanced solid tumors Advanced Hepatocellular Carcinoma Bladder Cancer Head and Neck Cancer Glioma/Astrocytoma Advanced Prostate Cancer Brain tumors Advanced Gastric Cance Metastatic Breast Cancer Metastatic Pancreatic Cancer |
||
|
| ||||||
| Approved | Soft-tisse and bone sarcomas |
|
||||
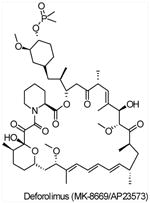
| AP23573 (Deforolimus,MK-8669) |
mTORC1 | Merck/Ariad | Phase I/II/III | Advanced malignancies Relapsed Hematologic Malignancies Progressive Glioma Endometrial Cancer Metastatic Breast Cancer Brain tumors Non-small cell lung cancer Prostate Cancer |
||
|
| ||||||
| AZD8055 | mTORC1/mTORC2 | AstraZeneca | Phase I/II | Advanced solid tumors Endometrial Carcinoma Lymphoma |
ND | |
|
| ||||||
| OSI-027 | mTORC1/mTORC2 | OSI | Phase I | Solid tumor Lymphoma |
ND | |
Information presented is compiled from company websites and from www.clinicaltrials.gov and www.fda.gov
The trial has been suspended due to low drug exposure.
ND, not disclosed
