Abstract
Methamphetamine (METH) is a neurotoxic drug. This study aimed to evaluate brain metabolite levels and cognitive function in young children with prenatal METH-exposure. 101 children ages 3-4 years were evaluated with neuropsychological tests and underwent proton magnetic resonance spectroscopy (1H-MRS) without sedation. Complete datasets from 49 METH-exposed and 49 controls who completed the neuropsychological test battery, and 38 METH-exposed and 37 controls with high-quality MR spectra are reported here. Despite similar physical characteristics (including head circumference), global cognitive function (on Stanford-Binet), parental education, intelligence, mood, and socioeconomic status, METH-exposed children had higher total creatine (tCr: +7%, p=0.003), N-acetyl compounds (NA: +4.3%, p=0.004) and glutamate+glutamine (GLX: +9.6%, p=0.02) concentrations in the frontal white matter, but lower myoinositol (MI: -7%, p=0.01) and MI/tCr (-7.5%, p=0.03) in the thalamus, than control children. The higher frontal white matter NA in the METH exposed children were due to the higher NA in the METH-exposed girls (+10.2%, p=0.0027), but not the boys (+0.8%) compared to sex-matched controls. Furthermore, the METH-exposed children had poorer performance on a visual motor integration (VMI) task, which correlated with lower MI in the thalamus (r=0.26, p=0.03). The higher NA, tCr and GLX concentrations suggest higher neuronal density or cellular compactness in the white matter, especially in the girls, whereas the lower MI suggests lower glial content in the thalamus of these METH-expose children. These findings combined with their poorer performance on VMI also suggest accelerated but aberrant neuronal and glial development in these brain regions.
Introduction
Methamphetamine (METH) is an addictive stimulant drug that primarily affects the dopaminergic and serotonergic systems in the brain, as shown in various animal models (Seiden et al. 1988; Melega et al. 1997; Marshall et al. 2007). The neurotoxic effects of METH also were observed in adult humans who abuse the drug, and were associated with abnormalities on neuropsychological tests, structural and functional neuroimaging studies (Ernst et al. 2000; Volkow et al. 2001; Chang et al. 2002; Paulus et al. 2003; Thompson et al. 2004; Chang et al. 2005; Jernigan et al. 2005; Nordahl et al. 2005; Sekine et al. 2006), as well as dopaminergic or serotonergic abnormalities in postmortem brains (Moszczynska et al. 2004; Kish et al. 2008). In adult METH users, the striatum which contains the highest density of dopaminergic synapses appears to be particularly vulnerable (Chang et al. 2007). Perhaps since the dopaminergic system regulates motor pathways and cognitive functions that require attention, METH users tended to show cognitive deficits related to psychomotor slowing, working memory and cognitive flexibility (Chang et al. 2002; Salo et al. 2007; van der Plas et al. 2008; Salo et al. 2009). However, little is known about possible brain alterations in children who were exposed to METH prenatally, and whether the drug might affect brain development.
Several studies in rats demonstrated that prenatal METH exposure may lead to greater axonal deformation and reduction in myelination in the optic nerves postnatally (Melo et al. 2006; Melo et al. 2008), as well as histological changes in the fetal brains (Cui et al. 2006). Furthermore, behavioral studies found prenatal METH exposure impairs development of postural motor movements (on the rotarod test) of rat pups during the first 3 postnatal weeks (Slamberova et al. 2006), but had little or no effect on learning or memory (on the Morris water maze) in adult male rats (Schutova et al. 2008). Preliminary MRI studies in children (ages 3-16 years) exposed to METH prenatally showed evidence for smaller basal ganglia and hippocampal structures, which correlated with some deficits in cognitive performance (Chang et al. 2004). The same study also found neurochemical changes, specifically elevated total creatine, on in vivo proton magnetic resonance spectroscopy (1H-MRS) (Smith et al. 2001).
The study aims to expand the prior evaluations of neurochemistry on MRS and cognition to a group of younger children with prenatal METH exposure. Both 1H-MRS and neuropsychological tests were performed in children ages 3 or 4 years, which is the earliest age that we could obtain reliable MRS data without sedation and neuropsychological testing with sufficient cooperation from the children. Based on prior observations, we hypothesized that: 1) children with prenatal METH exposure would show elevated total creatine compared to unexposed healthy children; 2) children with prenatal METH exposure would show poorer performance on some cognitive measures, including visual motor integration, attention and memory, compared to the unexposed children.
Methods and Materials
Research participants
144 children (52 METH-exposed, 92 unexposed controls) with similar socioeconomic backgrounds were screened as outpatients and 121 children (49 METH-exposed and 72 unexposed) were enrolled for this study. Each parent or legal guardian signed a written informed consent and completed detailed interviews regarding their medical and drug use histories and socioeconomic status. All children were evaluated thoroughly with birth record reviews, medical and neurological examination. 98 children also completed the neuropsychological tests. 101 completed the MR studies without sedation, but only 78 (81.6% METH exposed, 77.6% un-exposed) had acceptable MRS data (25 children required up to 4 repeat scanning sessions). Three more MRS datasets were excluded due to a quantitation problem in one and maternal family history of muscular dystrophy with maternal borderline intelligence quotient in two.
Inclusion criteria for the children: 1) male or female child of any ethnicity, ages 3 or 4 years; 2) parental or guardian willingness for the child to participate in the studies; 3) for the prenatal METH-exposed group: any maternal self-reported METH use during pregnancy of the child or positive meconium toxicology (screen+GC/MS confirmation) at birth of the child.
Exclusion criteria include: 1) a congenital or genetic neurological disorder; 2) prematurity (gestational age<36 weeks); 3) failure to thrive within first year of life; 4) overt TORCH infection at birth or a major neurological disorder since birth; 5) other contraindications for MR studies or severe claustrophobia. In addition, a child was excluded if the mother were: 1) age <17 years at the time of childbirth; 2) non-English speaking; 3) low cognitive functioning (National Adult Reading Test–estimated verbal IQ<80) or institutionalized for retardation; 4) seropositive for HIV-1 during pregnancy; 5) having a history of co-morbid psychiatric illness that may confound the outcome measures (e.g. schizophrenia, bipolar disorder or major depression not attributed to drug abuse or withdrawal); 6) having any medical condition during pregnancy that could cause alteration in the child's brain development; 8) having a history of drug dependence during pregnancy (except for METH in the METH-exposed group, or nicotine); light marijuana or alcohol use (<1 drink/day) were allowed.
Neuropsychological evaluations
The test battery includes measures of global cognitive functioning, with additional emphasis on tests of language development, visual motor integration and attention. These tests evaluated: 1) Global intelligence: Stanford-Binet Abbreviated Battery IQ (ABIQ) (Roid 2003), an estimated IQ based on verbal and non-verbal test performance. 2) Fluid reasoning: Stanford-Binet Routing Non-Verbal, which tests the child's ability to recognize patterns in object and picture placement (Roid 2003). 3) Vocabulary: Stanford-Binet Verbal, a test of spoken vocabulary for objects, pictures, and words (Roid 2003). 4) Naming and word retrieval: Expressive One Word Picture Vocabulary Test-Revised (EOWPVT-R), a naming task consisting of up to 143 items that assessed expressive language ability and memory retrieval of acquired knowledge (Gardner 1983). 5) Comprehension/ receptive vocabulary: Peabody Picture Vocabulary Test-Third Edition (PPVT-III), which consists of 175 test items designed to measure auditory receptive language (Sattler 1990). 6) Visual motor integration: Beery Test of Visual Motor Integration, in which the subjects were instructed to copy up to 24 geometric figures arranged in order of increasing difficulty (Sattler 1990). 7) Visual attention/visuomotor tasks: The Developmental Neuropsychological Assessment (NEPSY) Visual Attention subtest, which involved visual scanning and attention, and evaluated psychomotor speed (Sattler 1990; Spreen and Strauss 1998).
MRI
The MRI studies were performed on a Siemens Trio 3.0 Tesla scanner, while the children slept or watched a video. An 8-channel head array from Siemens/MRI Devices was used. The MRI began with a sagittal T1-weighted localizer (echo time/relaxation time or TE/TR=5/20 msec, 10-mm slice thickness, 2-mm gap, 256-mm field of view or FOV), followed by a 3D-magnetization prepared rapid acquisition by gradient echo (MP-RAGE, TE/inversion time (TI)/TR=491/1000/2200 msec, 1-mm isotropic resolution, 256-mm FOV, GRAPPA with 2-fold acceleration). Next, a fluid attenuated inversion recovery sequence (TE/TI/TR=99/2500/9750 msec, 3-mm slice thickness, no gap, 220-mm FOV, GRAPPA with 2-fold acceleration) was performed to further screen for any brain pathology.
Localized 1H-MRS
Voxel locations were prescribed from the MP-RAGE images. Spectroscopic data were collected from right frontal white matter (FWM), medial frontal gray matter at the anterior cingulate (AC), bilateral thalamus (THAL) and right basal ganglia (BG) (see Figure 1). Anatomical landmarks surrounding each voxel, as described in the Figure 1 legend, were used to provide guidance for consistent voxel placement. A standard Point RESolved Spectroscopy (PRESS) acquisition sequence (relaxation time/echo time =3000/30 msec, 48 averages, 2.5 min per location) was used. Metabolite concentrations were determined using a modified method that uses the unsuppressed water signal from each voxel as a reference (Ernst et al. 1993; Kreis et al. 1993). Briefly, the T2 decay of the unsuppressed water signal from the PRESS experiment was measured at 10 different TE and at 2 TR values to calculate metabolite concentrations corrected for the partial volume of cerebrospinal fluid. The spectral data were processed using the LCModel program (Provencher 2001), which yielded metabolite concentrations of N-acetyl compounds (NA), total creatine (tCr), choline-containing compounds (CHO), myoinositol (MI), and glutamate+glutamine (GLX). Furthermore, percent gray matter, white matter, and CSF within each voxel were determined using a customized program. These measures were used as covariates for metabolite concentrations, to correct for possible differences in gray-white matter proportion across subjects.
Figure 1.
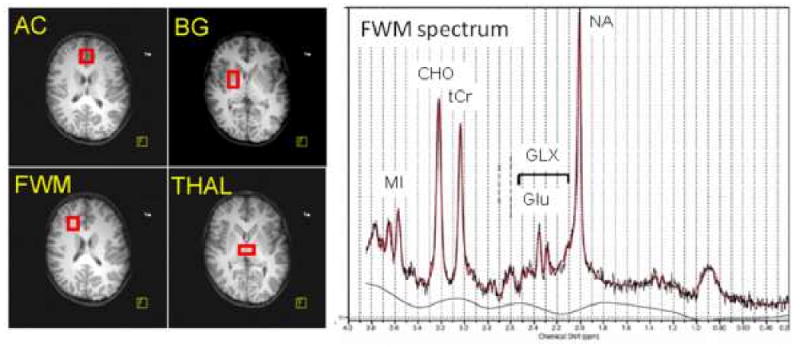
Left: Axial MR images showing the placement of the four voxels: 1) Anterior cingulate (AC), which contained primarily gray matter at the anterior and medial portions of both cingulate cortices across both hemispheres, and above the anterior portion of the head of caudate; 2) frontal white matter (FWM), which was confined to white matter in the right frontal lobe at the same level as the AC; 3) basal ganglia (BG), which was also obtained from the right hemisphere and contained the center portions of the putamen, the globus pallidus and some portion of the body of the caudate; 4) thalamus (THAL), which was confined to the gray matter structure and spanned across both hemispheres, slightly more posterior to the BG voxel. Right: A representative MR spectrum from the frontal white matter, showing well defined peaks for each of the brain metabolites measured (NA = N-acetylaspartate; GLX = glutamate+glutamine; Glu = glutamate; tCr = total creatine; CHO = choline-containing compounds; MI = myoinositol). The red line shows the integrated areas using LCModel.
There were no differences between subject groups in spectral quality (full width at half maximum or signal to noise ratio) in three of the four regions. In the anterior cingulate, unexposed controls had slightly greater line widths than the METH-exposed group (0.040±0.002 ppm vs. 0.034±0.002 ppm, p=0.03), possibly due to greater head motion during the scans.
Statistics
Statistical analyses were performed using Statistical Analysis Software or SAS, version 9.1 (SAS Institute Inc., Cary, NC). Brain metabolite concentrations and ratios were compared between groups using unpaired t-tests, and two-way analyses of variance were performed to evaluate METH-exposure-by-sex differences. One-way analysis of covariance was performed to control for potential confounding variables (demographic variables with p<0.25 in Table 1). Correlations between metabolite levels that showed significant group differences and clinical characteristics of the children or the mother/caregivers were performed using Pearson correlations (for variables that are normally distributed) or Spearman correlations (for variables that are non-normally distributed). In all analyses, a p-value<0.05 was considered significant.
Table 1.
Clinical characteristics of the research participants (Mean ± SEM)
| METH Exposed (n=49) |
Un-exposed (n=49) |
p-value | |
|---|---|---|---|
| Characteristics of the children | |||
| # of children (% males / % females) | 30(61%)/19(39%) | 27(55%)/22(45%) | 0.54 |
| Age (months) | 46.90 ± 1.07 | 45.17 ± 0.99 | 0.24 |
| Current Height (cm) | 98.11 ± 0.91 | 100.06 ± 0.90 | 0.13 |
| Current Weight (kg) | 16.42 ± 0.40 | 17.24 ± 0.41 | 0.16 |
| Current Head Circumference (cm) | 50.37 ± 0.21 | 50.63 ± 0.18 | 0.34 |
| Gestational age at birth (wks) | 38.74 ± 0.23 | 39.47 ± 0.18 | 0.01 |
| Activity, Pulse, Grimace, Appearance, and Respiration (APGAR) score at 5 min | 8.86 ± 0.08 | 9.03 ± 0.05 | 0.07 |
| Birth weight (kg) | 3.10 ± 0.11 | 3.48 ± 0.07 | 0.003 |
| Birth length (cm) | 49.58 ± 0.45 | 50.94 ± 0.97 | 0.22 |
| Maternal age and drug exposure | |||
| Age at time of child's birth (yrs) | 24.53 ± 0.99 | 26.06 ± 0.96 | 0.27 |
| #Trimesters used METH | 2.42 ± 0.14 | 0 | |
| #Trimesters used alcohol | 0.85 ± 0.21 | 0.21 ± 0.08 | 0.002 |
| #Trimesters used nicotine cigarettes | 2.05 ± 0.21 | 0.67 ± 0.17 | <0.0001 |
| #Trimesters used marijuana | 1.00 ± 0.22 | 0.12 ± 0.07 | <0.0001 |
Results
Clinical characteristics of the children (Table 1)
The two subject groups had similar age and gender proportion. There were also no group differences in the children's height, weight, or head circumference. However, METH-exposed children had slightly younger gestational age at birth and hence lower birth weight compared to controls. Of the METH-exposed children, 70% were exposed to METH during all three trimesters, 8% during two of the trimesters and 22% during the first trimester only. The mothers' self-reported mean METH usage during pregnancy was 0.32±0.09g/day (first trimester), 0.28±0.12g/day (second trimester), and 0.28±0.12g/day (third trimester). METH-exposed children were also exposed to more alcohol, nicotine and marijuana during more trimesters compared to controls: occasional alcohol use (typically<5 drinks/week): 13(27%) METH-exposed vs. 8(16%) control children; nicotine: 23(47%) of the METH-exposed children vs. 11(24%) of controls (11.03±1.6 vs. 10±3 cigarettes/day); light or occasional marijuana: 15(31%) METH-exposed vs. 4(8%) control children.
Primary caretaker or maternal factors and cognitive performance of children (Table 2)
Table 2.
Maternal or primary caretakers' characteristics and cognitive function of the children (Mean ± SEM)
| Meth Exposed (n=49) |
Un-exposed (n=49) |
p-value | |
|---|---|---|---|
| Socioeconomic factors of the primary caregiver (parent or legal guardian) | |||
| Biological mothers' education (years) | 12.19 ± 0.30 | 12.92 ± 0.29 | 0.082 |
| Primary care providers' education (years) | 12.96 ± 0.35 | 12.90 ± 0.28 | 0.89 |
| Estimate Verbal Intelligence Quotient | 99.96 ± 1.64 | 97.57 ± 1.21 | 0.24 |
| Beck Depression Inventory | 10.41 ± 2.05 | 10.53 ± 1.75 | 0.96 |
| Socioeconomic status (Hollingshead Index of Social Position) | 47.02 ± 2.74 | 47.50 ± 2.40 | 0.89 |
| Neuropsychological test performance of the children | |||
| Stanford-Binet Non-verbal (Fluid Reasoning) | 9.51 ± 0.41 | 9.47 ± 0.50 | 0.94 |
| Stanford-Binet Verbal (Vocabulary) | 8.70 ± 0.32 | 8.64 ± 0.35 | 0.89 |
| Stanford-Binet Verbal ABIQ | 93.41 ± 2.48 | 94.93 ± 1.99 | 0.63 |
| Expressive One Word Picture Vocabulary Test | 81.39 ± 2.13 | 81.68 ± 2.50 | 0.93 |
| Beery-Visual Motor Integration | 92.55 ± 2.01 | 100.88 ± 2.56 | 0.01 |
| Peabody Picture Vocabulary test-III | 91.43 ± 2.38 | 86.77 ± 2.36 | 0.17 |
| NEPSY Attention | 10.13 ± 0.33 | 10.39 ± 0.38 | 0.60 |
ABIQ: Abbreviated intelligence quotient
NEPSY: Developmental Neuropsychological Assessment – Visual Attention subtest
The biological mothers or primary caregivers of the two groups of children had similar education level, estimated verbal intelligence quotient (IQ), depressive symptoms on Beck depression scale, and socioeconomic status (Hollingshead Two Factor Index). On the neuropsychological tests, the METH-exposed children scored significantly lower on the Beery-Visual Motor Integration test compared to controls (92.6±2.0 vs. 100.9±2.6, p=0.01). No other group differences in cognitive function were observed.
MR spectroscopy findings
Table 3 shows the brain metabolite concentrations. Analysis of covariance was used to adjust for the percentage of gray matter in each voxel. METH-exposed children had significantly higher tCr (+7.0%. p=0.003), NA (+4.3%, p=0.004) and GLX (+9.6%, p=0.02) in the frontal white matter compared to controls (Figure 2). Although frontal white matter MI showed no group difference, the higher tCr, METH-exposed children led to their significantly lower MI/tCr (-9.6%, p=0.02) in frontal white matter compared to controls. Furthermore, in the thalamus, METH-exposed children had both significantly lower MI (-7.0%, p=0.01) and MI/tCr (-7.5%, p=0.03) compared to controls. No group differences were observed in the gray-white proportion in each of the brain regions measured (Table 3). Potential confounding characteristics (those with p<0.25) from Table 1 were examined using analysis of covariance. Since the two groups differed by birth weight, it was included as a covariate. The group difference for frontal white matter MI/tCr became less significant (p=0.08), but none of the other potential confounders significantly impacted MRS results in frontal white matter or in thalamus.
Table 3.
Brain metabolite concentrations in METH-exposed (n=38, 47.2±1.1 months) and unexposed children (n=37, 46.8±1.3 months) (mean ± SEM)
| Frontal white matter | Thalamus | |||||
|---|---|---|---|---|---|---|
| METH-exposed | Control | P-value* | Meth-exposed | Control | P-value* | |
| tCr | 5.78 ± 0.08 | 5.40 ± 0.10 | 0.003 | 6.81 ± 0.13 | 6.89 ± 0.15 | 0.61 |
| NA | 7.80 ± 0.09 | 7.48 ± 0.09 | 0.004 | 8.72 ± 0.14 | 8.87 ± 0.18 | 0.56 |
| CHO | 2.22 ± 0.06 | 2.07 ± 0.05 | 0.13 | 2.09 ± 0.04 | 2.10 ± 0.05 | 0.67 |
| MI | 3.82 ± 0.10 | 3.91 ± 0.10 | 0.92 | 4.25 ± 0.10 | 4.57 ± 0.10 | 0.01 |
| GLX | 8.59 ± 0.28 | 7.84 ± 0.17 | 0.02 | 10.71 ± 0.23 | 11.25 ± 0.24 | 0.16 |
| NA/tCr | 1.35 ± 0.01 | 1.39 ± 0.02 | 0.30 | 1.29 ± 0.02 | 1.29 ± 0.02 | 0.95 |
| CHO/tCr | 0.39 ± 0.01 | 0.38 ± 0.01 | 0.65 | 0.31 ± 0.01 | 0.31 ± 0.01 | 0.78 |
| MI/tCr | 0.66 ± 0.02 | 0.73 ± 0.01 | 0.02 | 0.62 ± 0.01 | 0.67 ± 0.02 | 0.03 |
| GLX/tCr | 1.49 ± 0.05 | 1.46 ± 0.03 | 0.62 | 1.57 ± 0.03 | 1.65 ± 0.03 | 0.31 |
| %GM | 19.2% | 22.9% | 83.3% | 85.4% | ||
| Basal ganglia | Frontal gray matter | |||||
| METH-exposed | Control | P-value* | METH-exposed | Control | P-value* | |
| tCr | 7.47 ± 0.14 | 7.71 ± 0.14 | 0.26 | 6.14 ± 0.07 | 6.08 ± 0.09 | 0.69 |
| NA | 7.93 ± 0.11 | 7.84 ± 0.14 | 0.66 | 7.83 ± 0.10 | 7.64 ± 0.12 | 0.30 |
| CHO | 1.87 ± 0.05 | 1.89 ± 0.05 | 0.81 | 1.43 ± 0.03 | 1.43 ± 0.03 | 0.77 |
| MI | 3.30 ± 0.12 | 3.24 ± 0.11 | 0.62 | 5.03 ± 0.08 | 4.93 ± 0.11 | 0.18 |
| GLX | 11.72 ± 0.24 | 12.03 ± 0.31 | 0.42 | 11.17 ± 0.21 | 11.12 ± 0.23 | 0.93 |
| NA/tCr | 1.07 ± 0.02 | 1.02 ± 0.01 | 0.07 | 1.28 ± 0.01 | 1.26 ± 0.02 | 0.60 |
| CHO/tCr | 0.25 ± 0.01 | 0.25 ± 0.01 | 0.20 | 0.23 ± 0.01 | 0.24 ± 0.01 | 0.60 |
| MI/tCr | 0.44 ± 0.02 | 0.42 ± 0.01 | 0.40 | 0.82 ± 0.02 | 0.81 ± 0.01 | 0.24 |
| GLX/tCr | 1.58 ± 0.03 | 1.56 ± 0.03 | 0.87 | 1.84 ± 0.04 | 1.84 ± 0.04 | 0.97 |
| %GM | 63.9% | 61.2% | 86.3% | 86.8% | ||
P-values after covarying (ANCOVA) for % gray matter from gray/white segmentation
Figure 2.
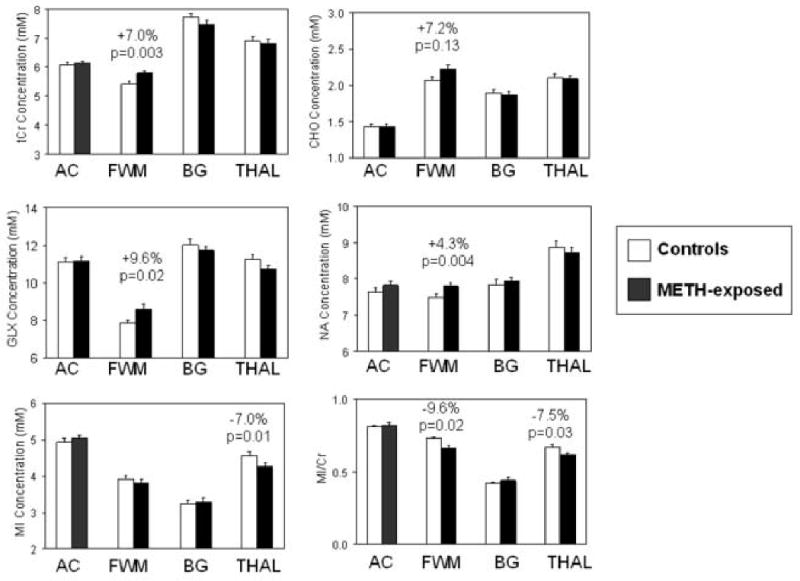
Bargraphs showing brain metabolite concentrations in four brain regions (also see Table 3). Higher tCr, NA, and GLX in the frontal white matter, but lower MI in the thalamus, are observed in METH-exposed children (black bars) compared to the unexposed children (white bars).
We also grouped our children into those who were exposed to METH for only one or two trimesters (n=13) and compare them to those who were exposed to METH for all three trimesters (n=25), and found no differences between these two groups in the elevated NA, Glx and tCr that we observed. However, those who were exposed to METH for all three trimesters showed higher frontal white matter choline compounds (+11.6%, p=0.038) than to those who were exposed to METH only for one or two trimesters.
Sex-differences on the effects of prenatal METH-exposure on brain metabolites
METH-by-sex interactions on NA (p=0.011) and MI/tCr (p=0.03) were observed in the frontal white matter (Figure 3). Specifically, METH-exposed girls showed higher frontal white matter NA (+10.2%, p=0.0027), whereas METH-exposed boys showed no difference (+0.8%) compared to sex-matched controls. Also in the frontal white matter, METH-exposed girls showed lower MI/tCr (-17.3%, p=0.0005), but METH-exposed boys showed non-significantly lower MI/tCr (-4.0%) compared to the sex-matched controls. The lower MI/tCr resulted primarily from a trend for greater elevation of tCr in the METH-exposed girls than the METH-exposed boys [girls: 6.01(METH) vs. 5.35(controls) mM; boys: 5.68(METH) vs. 5.45(controls) mM; METH-by-sex interaction p=0.11], and the slightly lower MI in the METH exposed girls than controls (3.65 vs. 3.95 mM) but similar MI levels in the METH-exposed and unexposed boys (3.89 vs. 3.88 mM). These sex-differences are not due to the reference (brain water) used for the concentration measurements, which showed no group difference (p=0.95).
Figure 3.
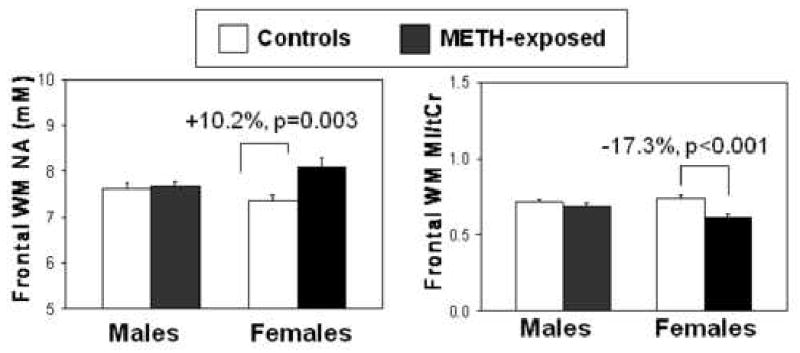
Bargraphs showing higher NA and lower MI/tCr only in the frontal white matter of the girls, but not the boys, exposed to METH (boys: n=23; girls: n=10) compared to those unexposed to the drug (boys: n=16, girls: n=15). METH-exposed x sex interaction: (NA, p=0.011; MI/tCr, p=0.03).
Exploratory analyses of correlations between brain metabolites and clinical variables
Lower thalamic MI was associated with poorer performance on the Beery-Visual Motor Integration scores (r=0.26, p=0.032) in both groups of children. Furthermore, socioeconomic status of the primary caregiver correlated positively with MI (r=0.28, p=0.023) and MI/tCr (r=0.35, p=0.004) in the thalamus of all the children. In addition, higher frontal white matter CHO was associated with poorer performance on the Expressive One Word Picture Vocabulary test scores (CHO: r=-0.28, p=0.024), and with shorter stature (r=-0.30, p=0.019) in all the children.
Despite the narrow age range, the two groups combined showed a trend for age-related increases in NA/tCr (r=0.24, p=0.058) in the frontal white matter, with the METH-exposed children showing consistently lower levels of this ratio across the ages. This is probably due to the consistently higher tCr across this age range in the METH-exposed group since no age-dependent change in NA was observed. No other age-related changes in brain metabolites across this small age range were seen in any of the brain regions.
Discussion
In a group of young children with prenatal METH-exposure, we observed abnormal brain metabolite concentrations in the frontal white matter and thalamus, as well as poorer performance on visual motor integration. Specifically, the METH-exposed children had higher tCr, NA, and GLX in the frontal white matter, as well as lower MI in the thalamus, than controls, despite similar parental education, intelligence, socioeconomic status and depressive symptoms. Our hypothesis of higher tCr in the METH-exposed children, which was based on the result from a prior small study of older (∼8 years) children exposed to METH prenatally (Smith et al. 2001), is validated in this relatively large group of young children (3>age<5 years) with or without METH-exposure prenatally.
Since the three metabolites NA, tCr and GLX are present in neurons, the higher concentrations in the white matter suggest increased axonal density or compactness in the METH-exposed children. This interpretation is consistent with prior reports of increased dendritic branching (Blaesing et al. 2001) and spine densities in the dorsolateral striatal regions of rodents after METH administration (Jedynak et al. 2007). Additionally, using diffusion tensor imaging, we found lower diffusivity in the frontal white matter of a subgroup of these children who were exposed to METH in-utero compared to controls (Cloak et al. 2009), which further supports our interpretation that the children exposed to METH in-utero have higher cellular density in the frontal white matter. Prior MRS studies of normally developing children (Kreis et al. 1993) and of healthy mice (postnatal days 5-20) (Weiss et al. 2009) found age-dependent increases in these three brain metabolites (NA, tCr and GLX). Therefore, the higher metabolite levels suggest an accelerated growth pattern in these young children with prenatal METH-exposure. Furthermore, the elevated frontal white matter tCr assessed with MRS was observed not only in children with prenatal METH-exposure (Smith et al. 2001), but also in children with prenatal cocaine exposure (Smith et al. 2001). However, it is unclear whether these analogous effects of METH and cocaine on brain metabolites are due to a mechanism common to psychostimulants, or to other more commonly co-abused substances (e.g. nicotine, alcohol or marijuana) that may have direct or indirect effects on brain metabolites.
The higher brain metabolites in the frontal white matter of METH or cocaine-exposed children also might have resulted from smaller brain volumes (Chang et al. 2004), which would lead to partial volume effects with higher % gray matter in the white matter voxels. However, our gray-white segmentation analyses demonstrated no group differences in the % gray matter in any of the voxels. Nevertheless, future detailed morphometric analyses of brain volumes in these children are needed to further address whether these children with prenatal METH-exposure have smaller or more compact cortical and subcortical brain volumes.
Since both tCr and CHO are significantly higher in glia than neurons (Brand et al. 1993), the elevated tCr and a trend for elevated CHO in the frontal white matter additionally suggest higher glial content. However, unlike adults who abuse METH (Ernst et al. 2000; Chang et al. 2007; Sung et al 2007) or adults with other acquired brain disorders (Chang et al. 2005) that often show glial activation with elevated glial marker MI, the METH-exposed children showed normal or lower MI in the thalamus. Therefore, the effects of METH on the immature developing brain, which has higher levels of MI prenatally than postnatally (Kreis et al. 1993), may be quite different and may not elicit a glial response such as that seen in the mature brains. Furthermore, unlike adult METH users that are typically studied within a short time from abstinence of the drug, our METH-exposed children had developing brains that are 3-4 years from an unknown amount of exposure to METH, which may be another reason for the lack of an apparent glial response. Lastly, children who were exposed to METH for all three trimesters had higher frontal white matter CHO than those exposed for two or less trimesters. The elevated CHO may be related to the greater prenatal alcohol exposure as well, since this has been well documented in both human and animal studies (Astley et al. 1995; Fagerlund et al. 2006). Future studies including more detail history of alcohol used in mothers who abused stimulants during pregnancy is needed.
We also found sex-differences in the brain metabolite abnormalities. The elevated frontal white matter NA was due primarily to the higher NA in the METH-exposed girls but relatively normal NA in the METH-exposed boys compared to the controls. Similarly, the METH-exposed girls but not boys showed significantly lower MI/tCr ratio. These findings suggest that in utero METH exposure has greater effects on the developing female fetal brain than the male fetal brain. These findings are contrary to a preclinical study that found prenatal METH-exposure led to greater reduction in myelin in male rats than in female rats, but higher rate of deformed axons and slighter lamellar separation in all animals (Melo et al. 2008). However, these investigators also found that prenatal METH exposure altered the levels of 3,4-dihydroxyphenylacetic acid (DOPAC) only in the retina of the female but not the male rats, with normal dopamine and tyrosine hydroxylase immunoreactivity in both sexes (Melo et al. 2006). Taken together, these data suggest prenatal METH-exposure may differentially affect the developing male and female brains.
The METH-exposed children, regardless of sex, also showed poorer performance on the Beery Visual motor integration task, which suggests that METH-exposure might have altered the motor or psychomotor neurodevelopment, possibly via the dopaminergic system in the fronto-striatal or thalmo-cortical pathways. Therefore, the possible accelerated growth with increased cellularity may be associated with an aberrant neural network in the frontal lobe in these children with prenatal METH-exposure. The poorer performance on the visual-motor integration task in these young 3-4 year old children with prenatal METH-exposure was also found in the prior small studies of older children (average age 8 years) with prenatal METH-exposure (Chang et al. 2004). Similarly, 3-week old rats (equivalent to that of our younger children) with prenatal METH exposure, regardless of sex, showed slower righting reflexes and more falls on the rotarod tests than unexposed rat pups, suggesting impaired development of postural motor movements (Slamberova et al. 2006). Since the brain metabolites alterations are found in the frontal brain region, more tests to assess other frontal lobe function, such as impulsivity and executive function, are needed.
The current study has several advantages compared to prior studies (Smith et al. 2001; Chang et al. 2004): a larger sample size, a much younger group of children with a narrow age range, stricter criteria that minimize other potential confounding conditions, and a higher magnetic field scanner. However, our METH-exposed children still had greater exposure to nicotine, marijuana and alcohol, and were born one-week earlier and hence smaller than the unexposed children at birth. These potential confounds were included as covariates in our analyses and the elevated frontal white matter tCr, NA and GLX of children exposed to METH in-utero remained or became more significant.
Longitudinal follow-up of these children with prenatal METH exposure, using structural MRI, MRS and more detailed neuropsychological evaluations will determine whether these brain metabolite abnormalities will persist, and how they would impact other aspects of cognition with further brain development in these children.
Acknowledgments
This study was supported by the National Institute of Health (1R01 DA21016; K24-DA16170; K02-DA16991; K01-DA21203; 1U54-NS56883; 1P20-RR11091). We also thank Drs. Helenna Nakama, Mary Ricardo-Dukelow, Daniel Alicata, and Michael Watters for some of the subject evaluations, Renat Yakupov and B. Stokes for some of the MRS data collection, James Armstrong for some of the MRS data processing, and Dr. Chris Derauf for referring some of the research participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astley SJ, Weinberger E, Shaw DW, Richards TL, Clarren SK. Magnetic resonance imaging and spectroscopy in fetal ethanol exposed Macaca nemestrina. Neurotoxicol Teratol. 1995;17(5):523–30. doi: 10.1016/0892-0362(95)00012-g. [DOI] [PubMed] [Google Scholar]
- Blaesing B, Nossoll M, Teuchert-Noodt G, Dawirs RR. Postnatal maturation of prefrontal pyramidal neurones is sensitive to a single early dose of methamphetamine in gerbils (Meriones unguiculatus) J Neural Transm. 2001;108(1):101–113. doi: 10.1007/s007020170101. [DOI] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102 1:16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psych. 2005;162(2):361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, Miller E. Perfusion MRI Abnormalities and Computerized Cognitive Deficits in Abstinent Methamphetamine Users. Psychiatry Res: Neuroimaging. 2002;114(2):65–79. doi: 10.1016/s0925-4927(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132(2):95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Cloak CC, Ernst T, Fujii L, Hedemark B, Chang L. Lower diffusion in white matter of children with prenatal methamphetamine exposure. Neurology. 2009 Apr 15; doi: 10.1212/01.wnl.0000346516.49126.20. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Sakata-Haga H, Ohta K, Nishida M, Yashiki M, Sawada K, Fukui Y. Histological brain alterations following prenatal methamphetamine exposure in rats. Congenit Anom (Kyoto) 2006;46(4):180–187. doi: 10.1111/j.1741-4520.2006.00126.x. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54(6):1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain. I: compartments and water. Journal of Magnetic Resonance. 1993;B102:1–8. [Google Scholar]
- Fagerlund A, Heikkinen S, Autti-Rämö I, Korkman M, Timonen M, Kuusi T, Riley EP, Lundbom N. Brain metabolic alterations in adolescents and young adults with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2006;30(12):2097–104. doi: 10.1111/j.1530-0277.2006.00257.x. [DOI] [PubMed] [Google Scholar]
- Gardner M. Expressive One-Word Picture Vocabulary Test-Revised. Novato, CA: Academic Therapy Publications; 1983. [Google Scholar]
- Jedynak JP, Uslaner JM, Esteban JA, Robinson TE. Methamphetamine-induced structural plasticity in the dorsal striatum. European Journal of Neuroscience. 2007;25(3):847–853. doi: 10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- Jernigan T, Gamst A, Archibald S, Fennema-Notestine C, Mindt M, Marcotte T, Heaton R, Ellis R, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psych. 2005;162(8):1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Fitzmaurice PS, Boileau I, Schmunk GA, Ang LC, Furukawa Y, Chang LJ, Wickham DJ, Sherwin A, Tong J. Brain serotonin transporter in human methamphetamine users. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1346-x. [DOI] [PubMed] [Google Scholar]
- Kreis R, Ernst T, Ross BD. Absolute quantitation of water and metabolites in the human brain. II: metabolite concentrations. Journal of Magnetic Resonance. 1993;B102:9–19. [Google Scholar]
- Kreis R, Ernst T, Ross BD. Development of the human brain: In Vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30:424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- Marshall JF, Belcher AM, Feinstein EM, O'Dell SJ. Methamphetamine-induced neural and cognitive changes in rodents. Addiction. 2007;102 1:61–69. doi: 10.1111/j.1360-0443.2006.01780.x. [DOI] [PubMed] [Google Scholar]
- Melega W, Raleigh M, Stout D, Lacan G, Huang S, Phelps M. Recovery of striatal dopamine function after acute amphetamine- and methamphetamine-induced neurotoxicity in the vervet monkey. Brain Res. 1997;766(1-2):113–120. doi: 10.1016/s0006-8993(97)00548-9. [DOI] [PubMed] [Google Scholar]
- Melo P, Moreno VZ, Vazquez SP, Pinazo-Duran MD, Tavares MA. Myelination changes in the rat optic nerve after prenatal exposure to methamphetamine. Brain Res. 2006;1106(1):21–29. doi: 10.1016/j.brainres.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Melo P, Pinazo-Duran MD, Salgado-Borges J, Tavares MA. Correlation of axon size and myelin occupancy in rats prenatally exposed to methamphetamine. Brain Res. 2008;1222:61–68. doi: 10.1016/j.brainres.2008.05.047. [DOI] [PubMed] [Google Scholar]
- Melo P, Pinazo-Duran MD, Salgado-Borges J, Tavares MA. Correlation of axon size and myelin occupancy in rats prenatally exposed to methamphetamine. Brain Research. 2008;1222:61–68. doi: 10.1016/j.brainres.2008.05.047. [DOI] [PubMed] [Google Scholar]
- Melo P, Rodrigues LG, Silva MC, Tavares MA. Effects of prenatal exposure to methamphetamine on the development of the rat retina. Ann N Y Acad Sci. 2006;1074:590–603. doi: 10.1196/annals.1369.058. [DOI] [PubMed] [Google Scholar]
- Moszczynska A, Fitzmaurice P, Ang L, Kalasinsky KS, Schmunk GA, Peretti FJ, Aiken SS, Wickham DJ, Kish SJ. Why is parkinsonism not a feature of human methamphetamine users? Brain. 2004;127(Pt 2):363–370. doi: 10.1093/brain/awh046. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, Moore CD, Kile S, Buonocore MH. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatr. 2005;62(4):444–452. doi: 10.1001/archpsyc.62.4.444. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biol Psychiatry. 2003;53(1):65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Provencher S. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001 Jun;14(4):260–264. doi: 10.1002/nbm.698. 2001. 14(4) [DOI] [PubMed] [Google Scholar]
- Roid GH. Stanford-Binet Intelligence Scales (SB5) Itasca, IL: Riverside Publishing Company; 2003. [Google Scholar]
- Salo R, Nordahl TE, Natsuaki Y, Leamon MH, Galloway GP, Waters C, Moore CD, Buonocore MH. Attentional control and brain metabolite levels in methamphetamine abusers. Biol Psychiatry. 2007;61(11):1272–1280. doi: 10.1016/j.biopsych.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry. 2009;65(8):706–709. doi: 10.1016/j.biopsych.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler J. Assessment of Children. Third. San Diego, CA: 1990. [Google Scholar]
- Schutova B, Hruba L, Pometlova M, Deykun K, Slamberova R. Cognitive functions and drug sensitivity in adult male rats prenatally exposed to methamphetamine. Physiol Res. 2008 doi: 10.33549/physiolres.931562. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Commins DL, Vosmer G, Axt K, Marek G. Neurotoxicity in dopamine and 5-hydroxytryptamine terminal fields: a regional analysis in nigrostriatal and mesolimbic projections. Ann N Y Acad Sci. 1988;537:161–172. doi: 10.1111/j.1749-6632.1988.tb42104.x. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H, Iyo M, Mori N. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry. 2006;63(1):90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- Slamberova R, Pometlova M, Charousova P. Postnatal development of rat pups is altered by prenatal methamphetamine exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(1):82–88. doi: 10.1016/j.pnpbp.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Gilbride K, Kuo J, Poland RE, Walot I, Ernst T. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107(2):227–231. doi: 10.1542/peds.107.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57(2):255–260. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: administration, norms and commentary. Second New York: Oxford University Press; 1998. [Google Scholar]
- Sung YH, Cho SC, Hwang J, Kim SJ, Kim H, Bae S, Kim N, Chang KH, Daniels M, Renshaw PF, Lyoo IK. Relationship between N-acetyl-aspartate in gray and white matter of abstinent methamphetamine abusers and their history of drug abuse: a proton magnetic resonance spectroscopy study. Drug Alcohol Depend. 2007;88(1):28–35. doi: 10.1016/j.drugalcdep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Thompson P, Hayashi K, Simon S, Geaga J, Hong M, Sui Y, Lee J, Toga A, Ling W, London E. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24(26):6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: A comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exp Neuropsychol. 2008:1–14. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Chang L, Wang G, Fowler J, Leonido-Yee M, Franceschi D, Sedler M, Gatley S, Hitzemann R, Ding Y, Logan J, Wong C, Miller E. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Weiss K, Melkus G, Jakob PM, Faber C. Quantitative in vivo (1)H spectroscopic imaging of metabolites in the early postnatal mouse brain at 17.6 T. MAGMA. 2009;22(1):53–62. doi: 10.1007/s10334-008-0142-2. [DOI] [PubMed] [Google Scholar]


