Abstract
Regulatory T cells (Tregs) play a key role in the prevention of acute graft-versus-host disease (aGVHD). To investigate the association between Treg subsets and aGVHD, we prospectively analysed T cell subsets in the allografts of 35 patients undergoing myeloablative unmanipulated haematopoietic stem cell transplantation. Multivariate analysis found that patients infused with less than 0·29 × 106/kg of CD4+CD25highCD45RA+CD62L+ T cells during transplantation exhibited an increased incidence of II−IV aGVHD [hazard ratio (HR) = 0·000, 95% CI = 0·000–0·106, P = 0·013]. Next, we compared the reconstitution characteristics of T cell subsets between haploidentical haematopoietic stem cell transplantation (HSCT) and sibling HSCT by collecting peripheral blood samples at regular intervals (days 30, 60 and 90) after transplantation. No significant differences were observed in the reconstitution of conventional T cells between haploidentical HSCT and sibling identical HSCT. However, total counts of recovered naiveTregs and CD62L+ naive Tregs from haploidentical HSCT were significantly lower compared to sibling identical HSCT; P-values were 0·045 and 0·021, respectively. Although total counts of conventional T cells in aGVHD patients reached similar levels compared to non-aGVHD patients before day 60 post-HSCT, total counts of naive Tregs and CD62L+ naive Tregs in aGVHD patients did not reach similar levels to non-aGVHD patients until 90 days post-HSCT. Taken together, our findings demonstrate that a large population of CD62L+ naive Tregs in allografts reduces the incidence of aGVHD. Further, development of aGVHD is related closely to the delayed reconstitution of the naive Treg population.
Keywords: acute graft-versus-host disease, allogeneic haematopoietic stem cell transplantation, immune reconstitution, immune tolerance, regulatory T cell
Introduction
Allogeneic haematopoietic stem cell transplantation (HSCT) provides potential curative therapy for patients with both malignant and non-malignant haematological diseases. However, acute graft-versus-host disease (aGVHD) remains a major obstacle to a more favourable therapeutic outcome of HSCT, as after HSCT patients tend to lose immune tolerance [1–4]. Immune tolerance is maintained by both central negative selection and a peripheral regulatory system. In HSCT, damage to the thymic epithelium by a pretransplantation-conditioning regimen and host-reactive T cells compromise negative selection in the thymus, which leads to the subsequent release of alloreactive T cells into the periphery. These cells recognize both major and minor histocompatibility antigens on recipient target cells, and an attack on these target cells results in aGVHD [1,2,5,6]. Therefore, the peripheral regulatory system plays a critical role in the establishment of tolerance between host tissue and donor-derived immune cells early after HSCT by suppressing the activity of pathogenic effector T lymphocytes.
Regulatory T cells (Tregs) play a key role in the maintenance of peripheral tolerance. Although a variety of Treg populations that ameliorate the development of aGVHD have been described, the majority of studies have focused on CD4+CD25+forkhead box P3 (FoxP3+) Tregs, which have been shown to suppress alloreactivity in a cell contact-dependent manner both in vitro and in vivo[7–11]. Patients who experienced aGVHD had a lower frequency of FoxP3+ Tregs compared to patients without aGVHD [12–15]; further, adoptive transfer of this subset protected against or reduced GVHD in both animal models and humans [16–19]. However, conflicting reports exist regarding the impact of Tregs on GVHD in humans. Indeed, several researchers have reported that Tregs do not influence the generation of aGVHD after HSCT [20,21]. These findings are supported by our previous work, which found that neither CD4+CD25+FoxP3+ Tregs in allografts nor their reconstitution after HSCT correlated with aGVHD [22].
In human peripheral blood, CD4+CD25+FoxP3+ Tregs reside mainly within the subset of CD4+ cells that highly express CD25 (CD25high). Hoffmann et al. [23] obtained CD4+CD25+FoxP3+ Tregs expanded from naive Tregs (CD45RA+ CD4+CD25high) in humans; furthermore, the transfer of naive Tregs was identified as the best strategy for adoptive therapy. As CD62L (l-selectin) is an important T cell homing receptor, CD62L+ Tregs enter preferentially into second lymphoid organs; in particular, entry into lymph nodes via high endothelial venules prevents aGVHD in target organs [24]. Ermann et al. [25] demonstrated that the adoptive transfer of CD62L+ but not CD62L- Tregs protected against GVHD in a murine model. Therefore, we hypothesize that a specific subset or subsets of CD4+CD25+FoxP3+ Tregs rather than the whole population are correlated with the development of aGVHD.
Previous clinical studies have identified that allograft characteristics such as infusion of increased numbers of total nucleated cells (TNC), CD34+ cells and CD3+ T cells are associated with the development of aGVHD. In contrast, a high percentage of FoxP3+ Tregs in allografts is associated with a reduced risk of aGVHD following HSCT with T cell depletion in vitro[26–29]. However, we found that the transfer of cells with a higher CD4/CD8 ratio and an increased population of CD4+CD45+CD62L+ in allografts increased the risk of aGVHD. No correlation between FoxP3+ Tregs and aGVHD risk was observed in our centre-performed unmanipulated allogeneic HSCT (without T cell depletion in vitro) [30,31]. Therefore, we sought to clarify whether the different reports of FoxP3+ Tregs in aGVHD were derived from different HSCT methodologies or from differences in Treg subpopulations.
This study analysed prospectively 35 patients undergoing unmanipulated allogeneic HSCT; we detected the absolute numbers and relative proportions of lymphocyte subsets in their allografts and found a strong correlation between the number of CD62L+ naive Tregs and the incidence of aGVHD. Our data also demonstrated that no differences were found in conventional T cell reconstitution between sibling identical HSCT and haploidentical HSCT. However, the total counts of naive Tregs and CD62L+ naive Tregs were decreased substantially following haploidentical HSCT. Analysis of the reconstitution of different T cell subsets between aGVHD patients and non-aGVHD patients showed that aGVHD patients exhibited a delayed reconstitution of naive and CD62L+ naive Tregs.
Materials and methods
Patients, samples and grouping
Peripheral blood samples were collected from 23 patients with malignant haematological diseases undergoing HSCT from July to September 2009 to detect the association between FoxP3+ Tregs and aGVHD and between FoxP3+ Tregs and CD4+CD25high Tregs. Blood samples were taken the day before transplantation and 30 days after transplantation. Additionally, we collected allograft samples from 35 patients with malignant haematological diseases undergoing HSCT (haploidentical HSCT n = 15, sibling HSCT n = 20) from December 2009 to February 2010. Donors received the granulocyte colony-stimulating factor (G-CSF) analogue filgrastim (Kirin Brewery Co, Tokyo, Japan) at 5 µg/kg daily for 5 days. On the fourth day bone marrow cells were harvested, and on the fifth day peripheral blood progenitor cells (PBPCs) were collected for the detection of allograft components. In addition, peripheral blood samples were collected at regular intervals (days 30, 60 and 90) after transplantation to study T cell reconstitution; however, only 20 patients who had not received donor lymphocyte infusion (DLI) until 90 days were included. Patient clinical history, pretransplantation-conditioning regimen, treatment, HSCT outcome, aGVHD symptoms and their donors' information are summarized in Table 1. All patients included in this study were enrolled in clinical protocols approved by the Institutional Review Board of Peking University Institute of Haematology. Written informed consents were obtained from both patients and their donors prior to sample collection.
Table 1.
Patient characteristics
| Characteristic | No. of patients (%) |
|---|---|
| Number | 35 |
| Median age, years | 31 (range 9–57) |
| Diagnosis | |
| Acute myeloid leukaemia | 15 (43) |
| Acute lymphoblastic leukaemia | 9 (26) |
| Myelodysplastic syndrome | 4 (11) |
| Chronic myeloid leukaemia | 4 (11) |
| Lymphoma | 3 (9) |
| Disease status, n | |
| Standard risk | 23 (66) |
| High risk | 12 (34) |
| Conditioning regimen | |
| Bu/Cy + ATG | 15 (43) |
| Bu/Cy | 11 (31) |
| Bu/Flu | 9 (26) |
| Stem cell source | |
| G-PB + G-BM | 33 (94) |
| G-PB only | 2 (6) |
| Donor median age, years | 40 (range 11–63) |
| Patient/donor HLA compatibility | |
| Matched | 20 (57) |
| Two locus mismatched | 7 (20) |
| Three locus mismatched | 8 (23) |
| Sex match, patient/donor | |
| Male/female | 15 (43) |
| Male/female | 7 (20) |
| Female/male | 8 (23) |
| Female/female | 5 (14) |
| Acute GVHD grade | |
| 0 to I | 25 (71) |
| II to IV | 10 (29) |
| Median follow-up days | 334 (range 275–360) |
| Engraftment | |
| Neutrophil engraftment, days (range) | 13 (range 9–23) |
| Platelet engraftment, days (range) | 14 (range 8–51) |
Bu: busulphan; Cy: cyclophosphamide; ATG: anti-human thymocyte immunoglobulin; G-PB: granulocyte colony-stimulating factor (G-CSF)-priming-peripheral blood progenitor cells; Flu: fludarabine.
Transplantation approach
Patients with human leucocyte antigen (HLA)-identical donors received either the myeloablative busulphan/cyclophosphamide (Bu/Cy) regimen or the busulphan/fludalabin (Bu/Flu) regimen. Patients with HLA mismatched or haploidentical donors received the Bu/Cy regimen and porcine anti-human thymocyte immunoglobulin (ATG). Prophylaxis for GVHD included treatment with cyclosporine A (CSA), short-term methotrexate (MTX) and mycophenolate mofetil (MMF), as described previously [3,4].
Engraftment, diagnosis and scoring of aGVHD
Neutrophil engraftment after transplantation was defined as an absolute neutrophil count (ANC) exceeding 0·5 × 109/l for 3 consecutive days; platelet engraftment was defined as a platelet count exceeding 20 × 109/l without transfusion for 7 consecutive days. Clinical aGVHD was graded according to standard criteria [32].
Treatment of aGVHD
aGVHD no less than grade II is usually treated with high-dose methylprednisolone, typically starting at a dose of 2 mg/kg per day. MTX and anti-CD25 monoclonal antibodies (mAb) were used to treat patients not fit for corticosteroid-therapy. aGVHD less than grade II is usually treated by the adjustment of the ongoing immunosuppressive drug therapy [33,34].
Flow cytometry
Samples were stained shortly after collection without further separation to minimize selective loss. Anti-CD4 peridinin chlorophyll protein (PerCP), anti-CD25 phycoerythrin (PE), anti-CD62L allophycocyanin (APC), anti-CD45RA fluorescein isothiocyanate (FITC), anti-FoxP3 PE, anti-CD25 APC and matched mouse isotype control antibodies (Becton-Dickinson, San Jose, CA, USA) were used. Intracellular analysis of FoxP3 expression was assessed using anti-FoxP3-PE (eBioscience, San Diego, CA, USA) and its mouse isotype control antibody; staining was performed after fixation and permeabilization according to the manufacturer's recommendations. Flow cytometry was performed using a BD FACSort.
Statistical analysis
Statistical analysis was performed using SPSS version 16·0. For non-normally distributed values, data were summarized by the median and ranges. For a two-related sample comparison of continuous variables, a two-sided Wilcoxon rank sum test was performed. For a two-unrelated sample comparison of continuous variables, a two-sided Mann–Whitney U-test was performed. For correlation analysis between non-normally distributed values, a Spearman correlation analysis was performed; for normally distributed values, a Pearson correlation analysis was performed. One-way univariate analysis using the Welch and Brown-Forsythe test was performed to select allograft components associated with aGVHD. Variates were grouped by median to analyse the association between subsets of allografts and aGVHD by Kaplan–Meier. Multivariate Cox proportional hazards models were assessed to avoid potential confounding factors. All factors with P < 0·05 in univariate analysis were included as covariates for testing interaction, other factors with P > 0·05 but that were associated closely with aGVHD were also included. Factors included were donor ages, conditioning regimen, HLA mismatch, dose of total nucleated cells, dose of CD34+ cells, dose of CD3+ T cells, dose of CD14+ cells, dose of CD4+ T cells, dose of CD8+ T cells and dose of FoxP3+ Treg subsets. The final multivariate models were built using a forward stepwise model selection approach. Repeated-measures analysis was performed to compare T cell reconstitution between haploidentical HSCT and sibling HSCT or between patients with aGVHD and those without aGVHD. P < 0·05 was considered statistically significant.
Results
CD4+CD25+FoxP3+ T cell frequencies decrease after HSCT, but do not correlate with aGVHD
A total of 23 patients with malignant haematological disease undergoing HSCT (haploidentical HSCT n = 16, sibling HSCT n = 7) were monitored for the detection of an association between FoxP3+ Tregs and aGVHD and an association between FoxP3+ Tregs and CD4+CD25high Tregs. The median age of patients was 39 years (range 22–49 years), and the median age of donors was 44 years (range 20–51 years). Sixteen (69·57%) of the 23 patients were considered standard risk and seven patients (30·43%) were considered high risk. While all 16 patients with HLA mismatched donors received the myeloablative Bu/Cy+ATG regimen, six of seven patients with HLA sibling donors received the myeloablative Bu/Cy regimen; the remaining patient with a HLA sibling donor received the Bu/Flu regimen. All patients exhibited stable neutrophil engraftment prior to day 30 post-HSCT. Nine patients (defined as the aGVHD group) suffered from aGVHD with a grade from II to IV. The median onset time was day 21 post-HSCT and ranged from days 17–89 following HSCT; all patients demonstrated a complete response (CR) to aGVHD following treatment. The other patients were defined as the non-aGVHD group (n = 14).
We compared the frequency of Tregs in patients prior to HSCT and 30 days after HSCT. Initial samples were collected at the time of hospital admission prior to the administration of any medicine associated with HSCT; post-HSCT samples were collected at day 30 to exclude any influence caused by DLI. Populations of CD4+CD25+FoxP3+ Tregs were not significantly different in aGVHD patients versus non-aGVHD patients either before HSCT (P = 0·477) or after HSCT (P = 0·250) (Fig. 1a); the same results were also observed for CD4+CD25high T cells (Fig. 1b). Both CD4+CD25+FoxP3+ Treg and CD4+CD25high T cell populations decreased after HSCT (Fig. 1c). FoxP3+ Tregs decreased from a median of 1·83% (range 0·15–6·08%) to a median of 0·91% (range 0·00–4·47%); similarly, CD4+CD25high T cells decreased from a median of 3·24% (range 0·25–10·28%) to a median of 1·73% (range 0·00–6·25%). To detect an association between FoxP3+ Tregs and CD4+CD25high T cells, correlation analysis was performed both before HSCT and after HSCT; a strong association was observed between FoxP3+ Tregs and CD4+CD25high T cells, and these results are presented in Fig. 1d,e.
Fig. 1.
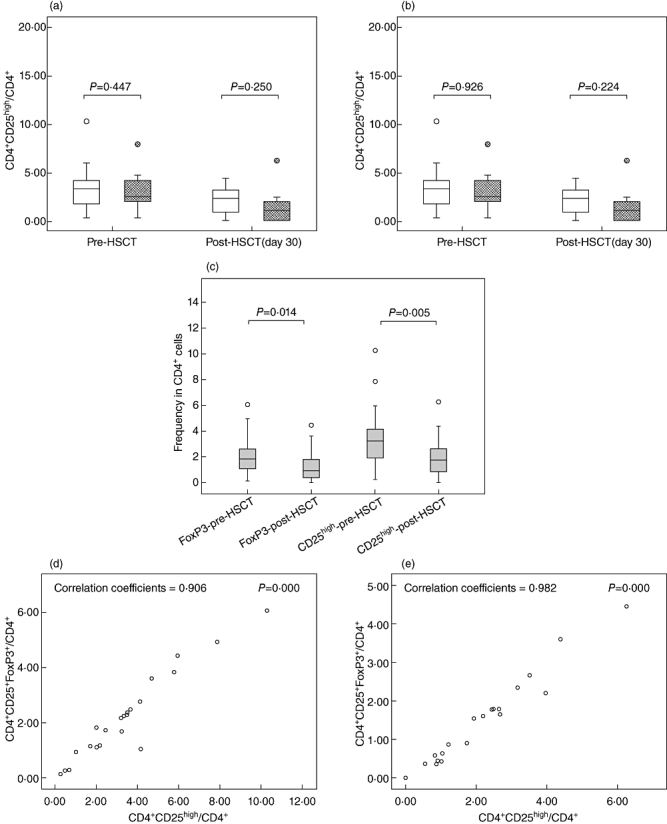
Frequencies of regulatory T cells (Tregs) pre-haematopoietic stem cell transplantation (HSCT) and post-HSCT. (a) Comparison of CD4+CD25+forkhead box P3 (FoxP3+) Treg frequencies between acute graft-versus-host disease (aGVHD) and non-aGVHD (non-aGVHD = white box, aGVHD = grey box). (b) Comparison of CD4+CD25high Treg frequencies between aGVHD and non-aGVHD (non-aGVHD = white box, aGVHD = grey box). (c) Frequencies of CD4+CD25+FoxP3+ and CD4+CD25high Tregs decrease post-HSCT. (d) Correlation analysis between CD4+CD25+FoxP3+ and CD4+CD25high Tregs pre-HSCT. (e) Correlation analysis between CD4+CD25+FoxP3+ and CD4+CD25high Tregs post-HSCT.
Absolute numbers of CD4+CD25highCD45RA+CD62L+ T cells in allografts are a strong predictor for the development of aGVHD
Naive Tregs were defined as described previously [23,35]; details are shown in Fig. 2. Table 2 depicts the subsets of cells present in the allografts of 35 HSCT patients. Patient and donor characteristics are listed in Table 1. Although the median onset time of aGVHD with a grade of II to IV was day 25 post-HSCT, onset ranged from days 15 to 45 post-HSCT. All patients exhibited a complete response (CR) to aGVHD following treatment. Univariate analysis showed that absolute CD4+CD45RA+CD62L+ T cell numbers, absolute CD4+CD25highCD45RA T cell numbers and absolute CD4+CD25highCD45RA+CD62L+ T cell numbers were statistically different between patients with aGVHD of grade 0–I and patients with aGVHD of grades II–IV; P-values were 0·038, 0·040 and 0·003, respectively. Variates were grouped by median of absolute numbers of those three subsets, respectively. The comparison of cumulative incidence of aGVHD between groups more than median and groups less than median were performed by Kaplan–Meier and a log-rank test as shown in Fig. 3. However, analysis of the correlation coefficients of various cell components in allografts revealed that many cell subsets showed statistically significant positive linear relationships with one another, indicating that these subsets could not be regarded as completely independent factors (Table 3). Therefore, their relative proportions were selected as factors for multivariate analysis. Finally, we found that patients who received less than 0·29 × 106/kg of CD4+CD25highCD45RA+CD62L+ T cells in their allograft exhibited an increased incidence of II–IV aGVHD [hazard ratio (HR) = 0·000, 95% confidence interval (CI) = 0·000–0·106, P = 0·013]).
Fig. 2.
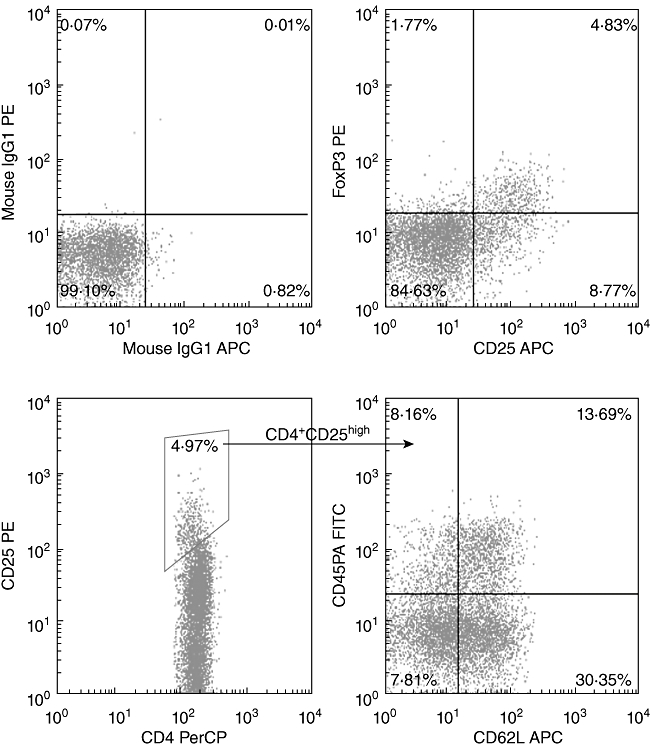
Flow cytometric analysis of regulatory T cell (Treg) frequencies. The frequency of each cell subset was determined as described in the Materials and methods. Cells were stained from allograft samples. (a) Isotype control. (b) CD4+CD25+ forkhead box P3 (FoxP3+) Tregs. (c) CD4+CD25high Tregs. (d) Subsets of CD4+CD25high Tregs.
Table 2.
Cellular composition of the mixed allografts of the granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood stem cell grafts and G-CSF-primed bone marrow grafts
| BM | PB | Total | |
|---|---|---|---|
| TNC × 108/kg | 2·61 (0·94–4·79) | 7·71 (2·16–14·59) | 9·90 (3·48–16·75) |
| CD3+ cells × 108/kg | 0·16 (0·05–0·41) | 2·19 (0·87–4·41) | 2·47 (1·06–4·69) |
| CD4+ cells × 108/kg | 0·08 (0·02–0·21) | 1·12 (0·49–2·27) | 1·22 (0·60–2·31) |
| CD8+ cells × 108/kg | 0·06 (0·03–0·79) | 0·94 (0·29–2·17) | 0·99 (0·36–2·32) |
| CD14+ cells × 108/kg | 0·08 (0·02–0·16) | 2·36 (0·71–5·27) | 2·44 (0·75–5·27) |
| CD34+ cells × 106/kg | 0·70 (0·21–2·26) | 2·79 (0·66–9·25) | 3·46 (1·45–9·90) |
| Naive T cells × 106/kg | 2·62 (0·09–10·61) | 40·16 (16·30–87·12) | 41·52 (17·95–92·19) |
| CD62L+ naive T cells × 106/kg | 0·37 (0·00–1·01) | 4·90 (2·14–13·65) | 5·62 (2·22–13·65) |
| CD4+CD25high T cells × 106/kg | 0·25 (0·00–2·68) | 8·86 (0·34–58·34) | 9·20 (0·39–58·84) |
| Naive Tregs × 105/kg | 0.70 (0·00–4·09) | 10·99 (4·67–55·35) | 11·26 (4·84–58·02) |
| CD62L+ naive Tregs × 105/kg | 0·09 (0·00–1·08) | 2·62 (0·20–35·65) | 2·88 (0·41–35·69) |
TNC: total nucleated cells; naive T cells: CD4+CD45RA+ T cells; CD62L+ naive T cells: CD4+CD45RA+CD62L+ T cells; naive regulatory T cells (Tregs): CD4+CD25highCD45RA T cells; CD62L+ naive Tregs: CD4+CD25high CD45RA+CD62L+ T cells; PB: peripheral blood; BM: bone marrow.
Fig. 3.
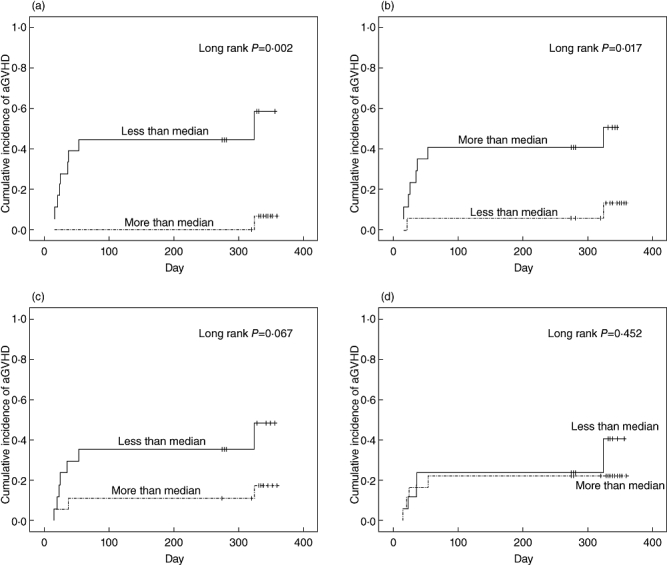
Association between the absolute number of cells in T cell subsets in allografts and acute graft-versus-host disease (aGVHD). (a) The incidence of aGVHD increased in patients infused with an absolute number of CD4+CD25high CD45RA+CD62L+ regulatory T cells (Tregs) less than 2·88 × 105/kg. (b) The incidence of aGVHD increased in patients infused with an absolute number of CD4+CD25highCD45RA Tregs less than 11·26 × 105/kg. (c) Infusion with absolute number of naive T cells more than 41·52 × 106/kg did not increase the incidence of aGVHD. (d) Infusion with absolute number of CD4+CD25high Tregs less than 9·20 × 106/kg did not increase the incidence of aGVHD.
Table 3.
Correlation coefficients for cell subsets within total allografts
| Cell subsets coefficients P-value | CD3+ | CD4+ | CD8+ | CD14+ | CD34+ | Naive | CD62L+ naive | Tregs | Naive Tregs | CD62L+ naive Tregs |
|---|---|---|---|---|---|---|---|---|---|---|
| TNC | 0·526 | 0·557 | 0·436 | 0·767 | 0·521 | 0·320 | 0·402 | 0·163 | 0·334 | 0·154 |
| 0·001 | 0·001 | 0·000 | 0·000 | 0·001 | 0·061 | 0·017 | 0·349 | 0·050 | 0·379 | |
| CD3+ | 0·872 | 0·802 | 0·423 | 0·336 | 0·624 | 0·562 | 0·241 | 0·344 | 0·247 | |
| 0·000 | 0·000 | 0·011 | 0·048 | 0·000 | 0·000 | 0·163 | 0·043 | 0·153 | ||
| CD4+ | 0·539 | 0·430 | 0·352 | 0·592 | 0·661 | 0·320 | 0·328 | 0·319 | ||
| 0·001 | 0·010 | 0·038 | 0·000 | 0·000 | 0·061 | 0·055 | 0·062 | |||
| CD8+ | 0·312 | 0·197 | 0·496 | 0·221 | 0·038 | 0·150 | 0·047 | |||
| 0·068 | 0·258 | 0·002 | 0·202 | 0·828 | 0·391 | 0·788 | ||||
| CD14+ | 0·439 | 0·204 | 0·325 | 0·046 | 0·231 | 0·031 | ||||
| 0·008 | 0·240 | 0·057 | 0·795 | 0·220 | 0·859 | |||||
| CD34+ | 0·229 | 0·344 | 0·259 | 0·374 | 0·181 | |||||
| 0·187 | 0·043 | 0·133 | 0·027 | 0·299 | ||||||
| Naive | 0·468 | 0·199 | 0·400 | 0·152 | ||||||
| 0·005 | 0·251 | 0·017 | 0·383 | |||||||
| CD62L+ naive | 0·583 | 0·712 | 0·639 | |||||||
| 0·000 | 0·000 | 0·000 | ||||||||
| Tregs | 0·501 | 0·773 | ||||||||
| 0·000 | 0·000 | |||||||||
| Naive Tregs | 0·564 | |||||||||
| 0·000 |
TNC: total nucleated cells; Tregs: regulatory T cells.
Kinetics of T cell reconstitution following HSCT
To investigate whether T cell subset reconstitution characteristics were influenced by transplantation method, we compared the kinetics of T cell reconstitution between haploidentical HSCT (n = 9) and sibling HSCT (n = 11). In total, seven patients (35%) suffered from aGVHD; of these, four received haploidentical HSCT (44·44%) and three received sibling identical HSCT (27·27%). Total counts of all T cell subsets declined after conditioning and were reduced significantly at day 30 post-HSCT both in haploidentical HSCT patients and sibling identical HSCT patients. Thereafter, these subsets expanded dramatically from days 30 to 60 in haploidentical HSCT patients. However, no differences were observed in any of the subsets between days 60 and 90 following haploidentical HSCT. There were no differences in the numbers of recovered CD3+, CD4+, CD8+ or Tregs between patients receiving haploidentical HSCT and those receiving sibling identical HSCT (Fig. 4a–d). In contrast, total counts of naive Tregs and CD62L+ naive Tregs in haploidentical HSCT patients were significantly lower compared to sibling identical HSCT patients (Fig. 4e–f). There were no differences in the frequency of total recovered T cells between haploidentical HSCT patients and sibling identical HSCT patients. The frequency of Tregs and Treg subsets increased from days 30 to 90 after HSCT in sibling identical HSCT patients; however, their frequency declined slightly in haploidentical HSCT patients from days 60 to 90 after HSCT (Fig. 4g–l).
Fig. 4.
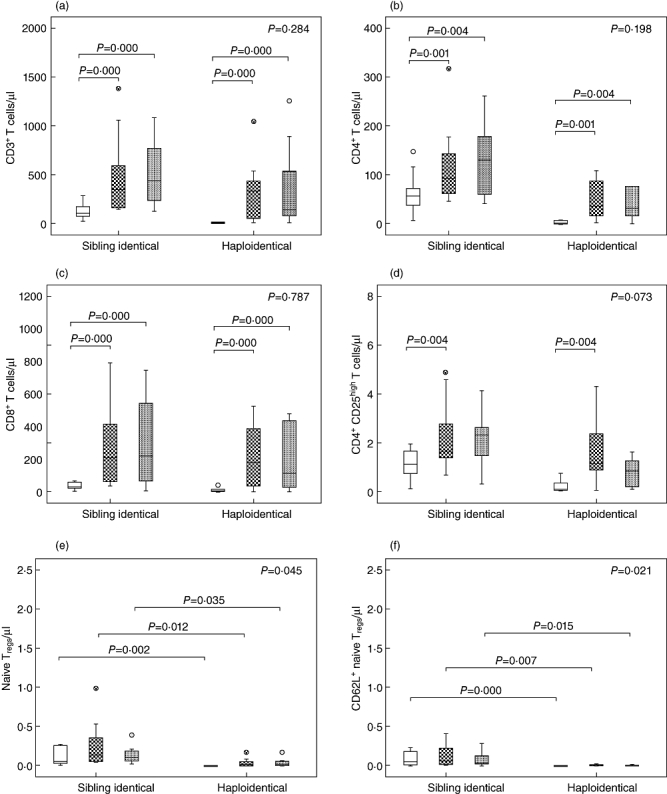
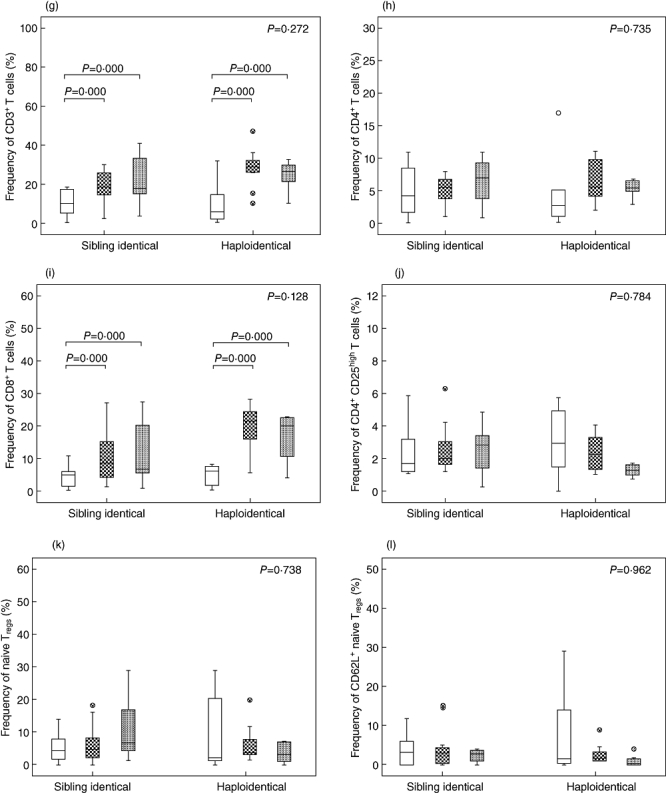
Comparison of T cell subset reconstitution between sibling identical haematopoietic stem cell transplantation (HSCT) and haploidentical HSCT. (a) Total counts of CD3+ cells. (b) Total counts of CD4+ cells. (c) Total counts of CD8+ cells. (d) Total counts of CD4+CD25high T cells. (e) Total counts of CD4+CD25highCD45RA T cells. (f) Total counts of CD4+CD25high CD45RA+CD62L+ T cells. (g) Frequencies of CD3+ cells in total lymphocytes. (h) Frequencies of CD4+ cells in total lymphocytes. (i) Frequencies of CD8+ cells in total lymphocytes. (j) Frequencies of CD4+CD25high T cells in total lymphocytes. (k) Frequencies of CD4+CD25highCD45RA T cells in CD4+CD25high T cells. (l) Frequencies of CD4+CD25high CD45RA+CD62L+ T cells in CD4+CD25high T cells. +30d = white box, +60d = grid box, +90d = spotted box.
Next, the same samples from the same 20 patients were used to compare T cell reconstitution between patients with aGVHD (n = 7) and those without aGVHD (n = 13). There were no differences in frequencies of all T cell subsets at any intervals between patients with aGVHD and those without aGVHD, while total counts of all T cell subsets were significantly lower in aGVHD patients compared to non-aGVHD patients on day 30-post HSCT. However, total counts of CD3+, CD4+, CD8+ and Tregs in aGVHD patients reached levels similar to non-aGVHD patients by day 60 post-HSCT. Despite this overall reconstitution, naive Tregs and CD62L+ naive Tregs in aGVHD patients were significantly lower compared to non-aGVHD patients by day 60 post-HSCT. By 90 days after HSCT, total counts of all T cell subsets in aGVHD patients reached similar levels to non-aGVHD patients (Fig. 5a–f). These data indicate that aGVHD is associated with delayed reconstitution of naive Tregs.
Fig. 5.
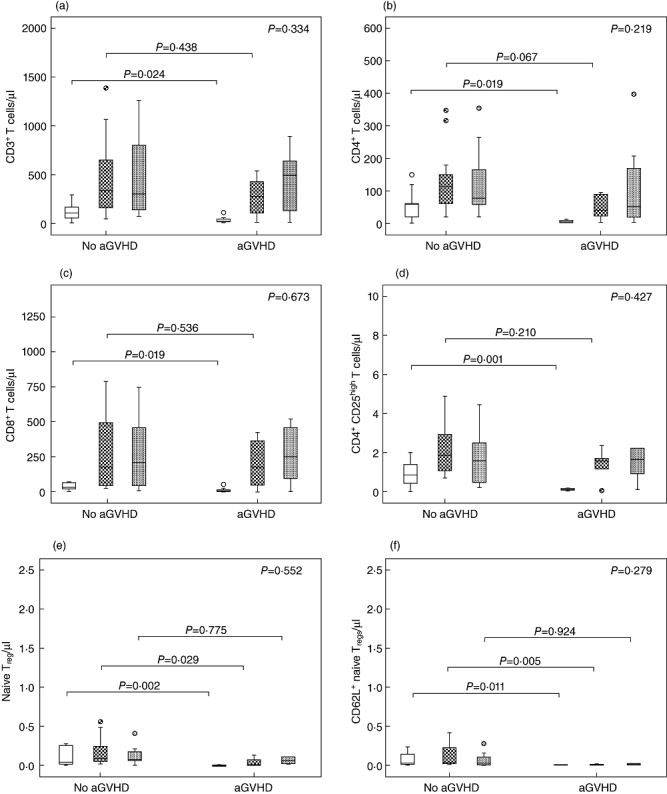
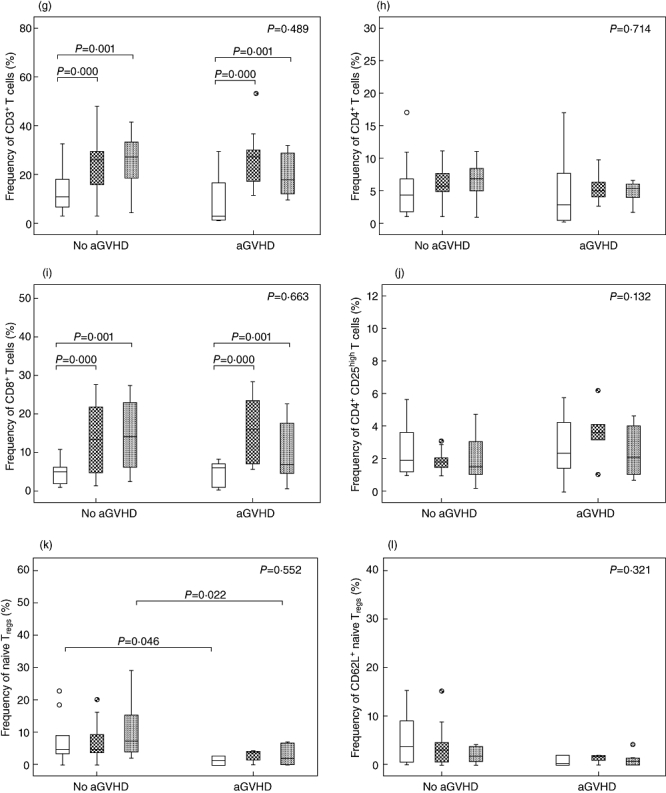
Comparison of T cell subset reconstitution between patients with and without acute graft-versus-host disease (aGVHD). (a) Total counts of CD3+ cells. (b) Total counts of CD4+ cells. (c) Total counts of CD8+ cells. (d) Total counts of CD4+CD25high T cells. (e) Total counts of CD4+CD25highCD45RA T cells. (f) Total counts of CD4+CD25high CD45RA+CD62L+ T cells. (g) Frequencies of CD3+ cells in total lymphocytes. (h) Frequencies of CD4+ cells in total lymphocytes. (i) Frequencies of CD8+ cells in total lymphocytes. (j) Frequencies of CD4+CD25high T cells in total lymphocytes. (k) Frequencies of CD4+CD25highCD45RA T cells in CD4+CD25high T cells. (l) Frequencies of CD4+CD25high CD45RA+CD62L+ T cells in CD4+CD25high T cells. +30d = white box, +60d = grid box,+90d = spotted box.
Discussion
Here, we provide the first indication that CD62L+ naive Tregs in allografts are a strong predictor of aGVHD. As the initial phase of T cell reconstitution depends primarily on the peripheral expansion of donor-derived T cells following myeloablative conditioning and allograft infusion, donor Tregs and Treg subsets can influence aGVHD development [36]. Therefore, the transfer of a high percentage of CD62L+ naive Tregs can promote reconstitution of naive Tregs and reduce the risk of aGVHD.
The Treg compartment in adult peripheral blood is comprised of naive as well as memory cells [35,37]; in fact, CD45RA+ naive Tregs account for approximately 42% of the total Treg population. The selective expansion of naive Tregsin vitro and in vivo has demonstrated sustained Treg expression of lymph node (LN) homing receptors [23,38]. As Tregs suppress alloreactivity in a cell contact-dependent manner, they require efficient entry to the priming sites of aGVHD. Therefore, it is not surprising that LN homing capacity plays an essential role in suppression of alloresponses in animal models of HSCT [8,24,25]. CD62L is an important T cell homing receptor that is crucial for T cell entry into LN. Ermann et al. demonstrated that CD62L+ Tregs home more efficiently to secondary lymphoid organs compared to their CD62L- counterparts; moreover, CD62L- T cells are less responsive to alloantigen stimulation compared to CD62L+ T cells [39]. Thus, CD62L+ naive Tregs with a strong homing capacity respond rapidly after encountering alloantigen in a target organ. It has been demonstrated that Treg entry into lymph nodes is crucial in inhibiting the initial expansion of donor-derived anti-host alloreactive T cells, which prevents aGVHD [40].
Previously, Chang et al. showed that the transfer of a large number of CD62L+ naive T cells during HSCT increased the risk of II–IV aGVHD [31]. Although the number of patients was limited, our results are consistent with this finding in univariate analysis but not in multivariate analysis, which indicates that CD62L+ naive Tregs may be a more sensitive object. We have also found naive Tregs to be a predictor using univariate analysis and the Kaplan–Meier method, but not in multivariate analysis. One plausible interpretation is that CD62L+, which distinguishes central memory and recently activated Tregs, was not used and confounded the findings regarding the total CD45RA+ naive Treg population; thus, to ensure population purity, CD62L+ should be used to identify Tregs that are capable of LN homing and are sensitive to alloresponses [25,41].
For conventional T cells, haploidentical immune reconstitution is comparable to sibling identical HSCT; however, differences have been observed in the reconstitution of Treg subsets. The reconstitution of Treg subsets following HSCT in our study is consistent with other reports reflecting an important role for lymphopenia-driven peripheral expansion of Tregs[42]. These data imply that peripheral tolerance is more important in the prevention of aGVHD than negative selection in the thymus early after HSCT [43,44]. CD4+ lymphopenia selectively induces Treg proliferation early after haploidentical HSCT, and the initial phase of Treg reconstitution is promoted by lymphopenia-related signals and stimulation by alloantigen [36]. Early after HSCT, Tregs have a higher chance to encounter abundant recipient alloantigens; however, with the reconstitution of CD4+ lymphocytes and other lymphocyte populations, the frequency of Treg subsets decreases, thus explaining why Treg frequency decreases after 30 days in patients with haploidentical HSCT. Other possibilities to explain the Treg reconstitution difference between haploidentical HSCT and sibling identical HSCT include that ATG, which is not used in sibling identical HSCT, decreases the expression of the main functional molecule cytotoxic T lymphocyte antigen (CTLA)-4 and down-regulates the expansion of FoxP3+ Tregs[45]. Additionally, high doses of CSA can cause a reduction in CD4+CD25high Tregs by decreasing interleukin (IL)-2 production [46–49]; further, MMF also inhibits Treg proliferation [50]. In our study, the haploidentical HSCT patients were treated with higher doses of CSA and MMF for a longer period of time compared to sibling identical HSCT patients. Our data were similar to findings that the frequencies of FoxP3+ Tregs and CD4+CD25high Tregs in patients undergoing HSCT were much lower compared to frequencies prior to HSCT (Fig. 1c). Further, the differences in immune reconstitution between aGVHD patients and non-aGVHD patients indicate strongly that the development of aGVHD is correlated highly with the delayed reconstitution of naive Tregs.
In summary, our data suggest that naive CD62L+ Treg content in allografts can predict the risk of aGVHD, and that impaired naive Treg reconstitution probably induces aGVHD. This study demonstrates a clinical association between aGVHD and CD62L+ naive Tregs. However, further studies will be required to investigate whether functional alteration of Treg subsets exists after allo-HSCT. In particular, longitudinal follow-up studies of post-transplantation patients will be required to establish whether the infusion of large numbers of CD62L+ naive Treg correlates with the prevention of cGVHD or with an increased incidence of relapse.
Acknowledgments
This work was supported by the National Outstanding Young Scientist's Foundation of China (grant no. 30725038), National Natural Science Foundation of China (grant no. 30971292 and 30800485) and the Program for Innovative Research Team in University (IRT0702). American Journal Experts (http://www.journalexperts.com) provided editorial assistance with the manuscript.
Disclosure
All the authors declare no competing financial interests.
References
- 1.Socie G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114:4327–36. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hess AD. Modulation of graft-versus-host disease: role of regulatory T lymphocytes. Biol Blood Marrow Transplant. 2006;12:13–21. doi: 10.1016/j.bbmt.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Lu DP, Dong L, Wu T, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006;107:3065–73. doi: 10.1182/blood-2005-05-2146. [DOI] [PubMed] [Google Scholar]
- 4.Huang XJ, Liu DH, Liu KY, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant. 2006;38:291–7. doi: 10.1038/sj.bmt.1705445. [DOI] [PubMed] [Google Scholar]
- 5.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 6.Clave E, Busson M, Douay C, et al. Acute graft-versus-host disease transiently impairs thymic output in young patients after allogeneic hematopoietic stem cell transplantation. Blood. 2009;113:6477–84. doi: 10.1182/blood-2008-09-176594. [DOI] [PubMed] [Google Scholar]
- 7.Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood. 2007;109:827–35. doi: 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- 8.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–9. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 9.Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews K, Lim Z, Afzali B, et al. Imbalance of effector and regulatory CD4 T cells is associated with graft versus-host disease after hematopoietic stem cell transplantation using a reduced intensity conditioning regimen and alemtuzumab. Haematologica. 2009;94:956–66. doi: 10.3324/haematol.2008.003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fondi C, Nozzoli C, Benemei S, et al. Increase in FOXP3+ regulatory T cells in GVHD skin biopsies is associated with lower disease severity and treatment response. Biol Blood Marrow Transplant. 2009;15:938–47. doi: 10.1016/j.bbmt.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Di Biaso I, Di Maio L, Bugarin C, et al. Regulatory T cells and extracorporeal photochemotherapy: correlation with clinical response and decreased frequency of proinflammatory T cells. Transplantation. 2009;87:1422–5. doi: 10.1097/TP.0b013e3181a27a5d. [DOI] [PubMed] [Google Scholar]
- 14.Rieger K, Loddenkemper C, Maul J, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107:1717–23. doi: 10.1182/blood-2005-06-2529. [DOI] [PubMed] [Google Scholar]
- 15.Mielke S, Rezvani K, Savani BN, et al. Reconstitution of FOXP3+ regulatory T cells (Tregs) after CD25-depleted allotransplantation in elderly patients and association with acute graft-versus-host disease. Blood. 2007;110:1689–97. doi: 10.1182/blood-2007-03-079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trzonkowski P, Bieniaszewska M, Juscinska J, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127− T regulatory cells. Clin Immunol. 2009;133:22–6. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Zhang L, Tang J, Jiang S, Wang X. Adoptive transfer of transplantation tolerance mediated by CD4+CD25+ and CD8+CD28− regulatory T cells induced by anti-donor-specific T-cell vaccination. Transplant Proc. 2008;40:1612–17. doi: 10.1016/j.transproceed.2008.02.079. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen VH, Shashidhar S, Chang DS, et al. The impact of regulatory T cells on T-cell immunity following hematopoietic cell transplantation. Blood. 2008;111:945–53. doi: 10.1182/blood-2007-07-103895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao T, Soto A, Zhou W, et al. Ex vivo expanded human CD4+CD25+Foxp3+ regulatory T cells prevent lethal xenogenic graft versus host disease (GVHD) Cell Immunol. 2009;258:65–71. doi: 10.1016/j.cellimm.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Noel G, Bruniquel D, Birebent B, et al. Patients suffering from acute graft-versus-host disease after bone-marrow transplantation have functional CD4+CD25hiFoxp3+ regulatory T cells. Clin Immunol. 2008;129:241–8. doi: 10.1016/j.clim.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Noel G, Bruniquel D, DeGuibert S, et al. Regulatory CD4+CD25hi T cells conserve their function and phenotype after granulocyte colony-stimulating factor treatment in human hematopoietic stem cell transplantation. Hum Immunol. 2008;69:329–37. doi: 10.1016/j.humimm.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Lu SY, Huang XJ, Liu KY, Liu DH, Xu LP. CD4+CD25-CD69+ T cells is a novel subset of regulatory T cells involved in the prevention of acute graft-versus-host disease in human. Blood. 2010;116:536. [Google Scholar]
- 23.Hoffmann P, Eder R, Boeld TJ, et al. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006;108:4260–7. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- 24.Taylor PA, Panoskaltsis-Mortari A, Swedin JM, et al. L-Selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 2004;104:3804–12. doi: 10.1182/blood-2004-05-1850. [DOI] [PubMed] [Google Scholar]
- 25.Ermann J, Hoffmann P, Edinger M, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220–6. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 26.Przepiorka D, Smith TL, Folloder J, et al. Risk factors for acute graft-versus-host disease after allogeneic blood stem cell transplantation. Blood. 1999;94:1465–70. [PubMed] [Google Scholar]
- 27.Zaucha JM, Gooley T, Bensinger WI, et al. CD34 cell dose in granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell grafts affects engraftment kinetics and development of extensive chronic graft-versus-host disease after human leukocyte antigen-identical sibling transplantation. Blood. 2001;98:3221–7. doi: 10.1182/blood.v98.12.3221. [DOI] [PubMed] [Google Scholar]
- 28.Urbano-Ispizua A, Rozman C, Pimentel P, et al. Risk factors for acute graft-versus-host disease in patients undergoing transplantation with CD34+ selected blood cells from HLA-identical siblings. Blood. 2002;100:724–7. doi: 10.1182/blood-2001-11-0057. [DOI] [PubMed] [Google Scholar]
- 29.Rezvani K, Mielke S, Ahmadzadeh M, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–7. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo XH, Chang YJ, Xu LP, Liu DH, Liu KY, Huang XJ. The impact of graft composition on clinical outcomes in unmanipulated HLA-mismatched/haploidentical hematopoietic SCT. Bone Marrow Transplant. 2009;43:29–36. doi: 10.1038/bmt.2008.267. [DOI] [PubMed] [Google Scholar]
- 31.Chang YJ, Zhao XY, Huo MR, Huang XJ. Expression of CD62L on donor CD4(+) T cells in allografts: correlation with graft-versus-host disease after unmanipulated allogeneic blood and marrow transplantation. J Clin Immunol. 2009;29:696–704. doi: 10.1007/s10875-009-9293-9. [DOI] [PubMed] [Google Scholar]
- 32.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Huang XJ, Jiang Q, Chen H, et al. Low-dose methotrexate for the treatment of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;36:343–8. doi: 10.1038/sj.bmt.1705034. [DOI] [PubMed] [Google Scholar]
- 34.Lu J, Xu LP, Huang XJ, et al. [Treatment of severe steroid-refractory graft-versus-host disease with IL-2R alpha chain (CD25) monoclonal antibody] Zhonghua Yi Xue Za Zhi. 2003;83:216–19. [PubMed] [Google Scholar]
- 35.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest. 2005;115:1953–62. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuoka K, Kim HT, McDonough S, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2010;120:1479–93. doi: 10.1172/JCI41072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seddiki N, Santner-Nanan B, Tangye SG, et al. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood. 2006;107:2830–8. doi: 10.1182/blood-2005-06-2403. [DOI] [PubMed] [Google Scholar]
- 38.Tang Q, Adams JY, Tooley AJ, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foster AE, Marangolo M, Sartor MM, et al. Human CD62L− memory T cells are less responsive to alloantigen stimulation than CD62L+ naive T cells: potential for adoptive immunotherapy and allodepletion. Blood. 2004;104:2403–9. doi: 10.1182/blood-2003-12-4431. [DOI] [PubMed] [Google Scholar]
- 40.Carlson MJ, Fulton LM, Coghill JM, et al. L-selectin is dispensable for T regulatory cell function postallogeneic bone marrow transplantation. Am J Transplant. 2010;10:2596–603. doi: 10.1111/j.1600-6143.2010.03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Chua KS, Guimond M, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11:1238–43. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 43.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–77. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Cozzo C, Larkin J, III, Caton AJ. Cutting edge: self-peptides drive the peripheral expansion of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:5678–82. doi: 10.4049/jimmunol.171.11.5678. [DOI] [PubMed] [Google Scholar]
- 45.Ruzek MC, Waire JS, Hopkins D, et al. Characterization of in vitro antimurine thymocyte globulin-induced regulatory T cells that inhibit graft-versus-host disease in vivo. Blood. 2008;111:1726–34. doi: 10.1182/blood-2007-08-106526. [DOI] [PubMed] [Google Scholar]
- 46.Coenen JJ, Koenen HJ, van Rijssen E, Hilbrands LB, Joosten I. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood. 2006;107:1018–23. doi: 10.1182/blood-2005-07-3032. [DOI] [PubMed] [Google Scholar]
- 47.Coenen JJ, Koenen HJ, van Rijssen E, et al. Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4+ CD25+ FoxP3+ T cells. Bone Marrow Transplant. 2007;39:537–45. doi: 10.1038/sj.bmt.1705628. [DOI] [PubMed] [Google Scholar]
- 48.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–74. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 49.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–23. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 50.Lim DG, Koo SK, Park YH, et al. Impact of immunosuppressants on the therapeutic efficacy of in vitro-expanded CD4+CD25+Foxp3+ regulatory T cells in allotransplantation. Transplantation. 2010;89:928–36. doi: 10.1097/TP.0b013e3181d3c9d4. [DOI] [PubMed] [Google Scholar]


