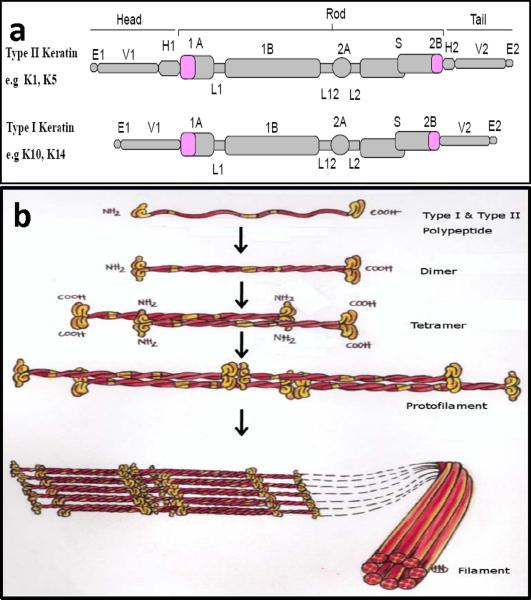
Figure 2. Molecular structure and assembly of keratin intermediate filaments (KIF).
(a) Schematic representation of type I and type II keratin polypeptide domain structural organization. Of the 54 different human keratin genes, each keratin molecule consists of a central alpha helical rod domain which is composed of four helical segments, 1A, IB, 2A and 2B that are interrupted by three flexible non-helical linker domains L1, L12 and L2. The rod domain begins and ends with highly conserved sequence motifs, helix initiation (HIP) and helix termination (HTP) peptides and is flanked by head and tail domains, respectively, (b) Keratin intermediate filaments assembly; Keratin polymerization obligatorily begins with the formation of coiled-coil obligate heterodimer structures involving winding around each other of the central rod domains of type I and type II polypeptides, a requirement underlying the pair wise transcriptional regulation of keratin genes in vivo. The heterodimers then associate (side-by-side) and assemble in an overlapping staggered and antiparallel fashion to form stable tetramers. Tetramers then associate end-to-end to form protofilaments and finally, four protofibrils laterally build keratin intermediate filaments. Each filament contains approximately eight protofilaments wound around each other in a rope-like structure, forming the 10–12 nm wide KIF network. (Reproduced from [133] with permission from the publisher)