Table 1.
Catalyst Systems used for the Coupling of 4-Chloroazaindole and N–Methylpiperazine
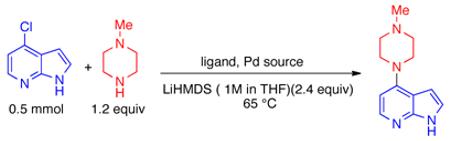
| entry | Pd source (mol %) |
ligand (mol %) |
time | yield |
|---|---|---|---|---|
| 1 | P1 (0.5) | L1 RuPhos (0.5) | 30 min | 94% |
| 2 | P2 (0.5) | L2 SPhos (0.5) | 30 min | 81% |
| 3 | P3 (0.5) | L3 XPhos (0.5) | 30 min | 90% |
| 4 | P4 (0.5) | L4 t-BuXPhos (0.5) | 30 min | 0% |
| 5 | Pd(OAc)2 (0.5) | L1 RuPhos (1) | 30 min | 80% |
| 6 | Pd2dba3 (0.25) | L1 RuPhos (1) | 30 min | 33% |
| 7 | Pd(OAc)2 (1) | Rac-BINAP (2) | 4 h | 0% |
| 8 | Pd(OAc)2 (1) | Xantphos (2) | 4 h | 0% |
Conditions: 4-chloroazaindole (0.5 mmol), N-methylpiperazine (0.6 mmol), LiHMDS (1.2 mmol, 1 M in THF).
