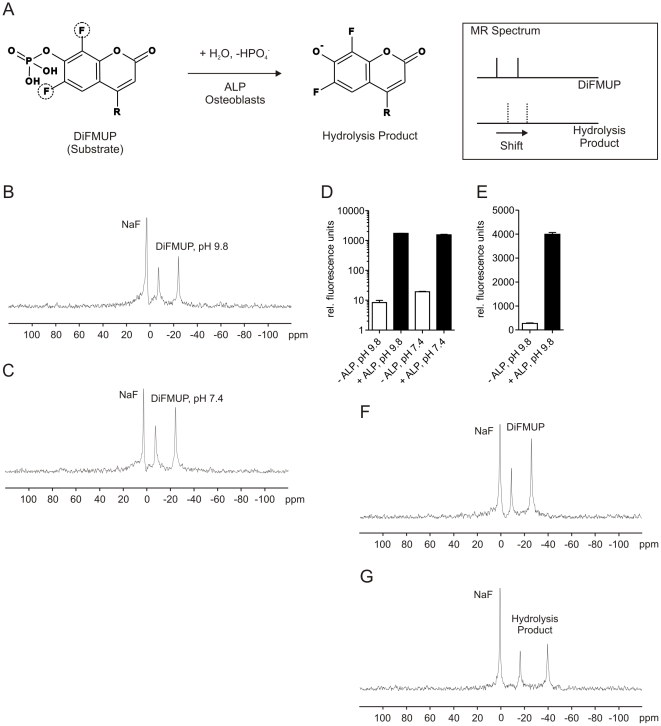
Figure 1. Basic concept and specific detection of the ALP substrate DiFMUP by magnetic resonance spectroscopy.
A: In this study, imaging is based on the use of DiFMUP. This small imaging molecule prototype contains two MR-detectable fluorine atoms in direct vicinity to a phosphate group. Prior to hydrolyzation by ALP, DiFMUP is expected to yield a spectrum characteristic of two discrete signals from the fluorine atoms since the molecule is not symmetrical, and hence the fluorine atoms exist in distinct chemical environments. If ALP is present and catalyzes the exchange of the phosphate to a hydroxy group, a significant change in the environment next to the fluorine atoms occurs and is expected to result in a chemical shift variation of the fluorine signals that is detectable by 19FMRSI. An added advantage of DiFMUP is the fluorescent property of its hydrolysis product; this allows for the convenient measurement of DiFMUP activation. This feature is not essential for the presented imaging paradigm, however it is used to monitor ALP activity in several subsequent figures. B: The two-peak MR signal of DiFMUP at alkaline pH 9.8 relative to a sodium fluoride (NaF) standard. C: An identical DiFMUP spectrum characteristic was observed under physiological pH 7.4 conditions. D and E: The hydrolysis product of DiFMUP exhibits fluorescence properties, which allows one to record DiFMUP activation. Substantial activation of 50 µM (D) and 5 mM (E) DiFMUP in the presence of ALP was seen in both alkaline and physiological solutions. F and G represent MR spectra of DiFMUP and its hydrolysis product, respectively. The discrete spectrum of the hydrolysis product is seen including resonances from non-equivalent fluorine atoms at −17 ppm and −41 ppm relative to the sodium fluoride reference resonance.