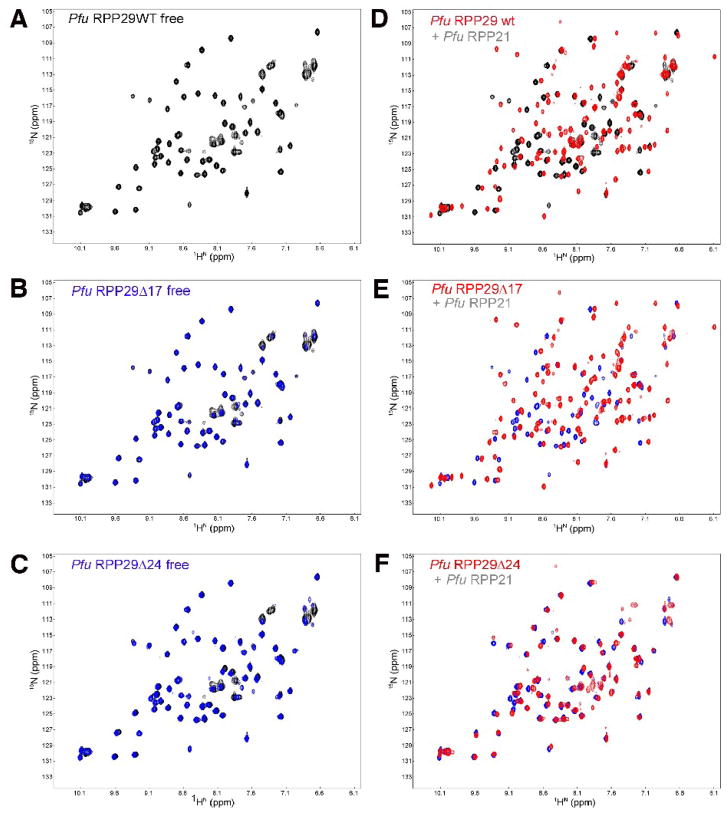
Figure 4.
NMR-based binding assay of Pfu RPP29 N-terminal deletion derivatives to RPP21. Both RPP29Δ17 (C) and RPP29Δ24 (E) adopt the same structured core as the wild type (A), indicated by the almost identical two-dimensional 1H-15N correlation spectra of the free 15N-labeled proteins. Titration of unlabeled RPP21 into 15N-labeled RPP29 induces dramatic spectral changes in the spectra of RPP29WT (B) and RPP29Δ17 (D), but not in the spectrum of RPP29Δ24 (F). In panels (C) and (E) the spectra of the free RPP29 derivative protein (blue) is overlaid on that of the free full-length protein (black). In panels (B), (D) and (F) the spectrum of each RPP29 variant is compared in the absence (back/blue) and presence (red) of equimolar RPP21.