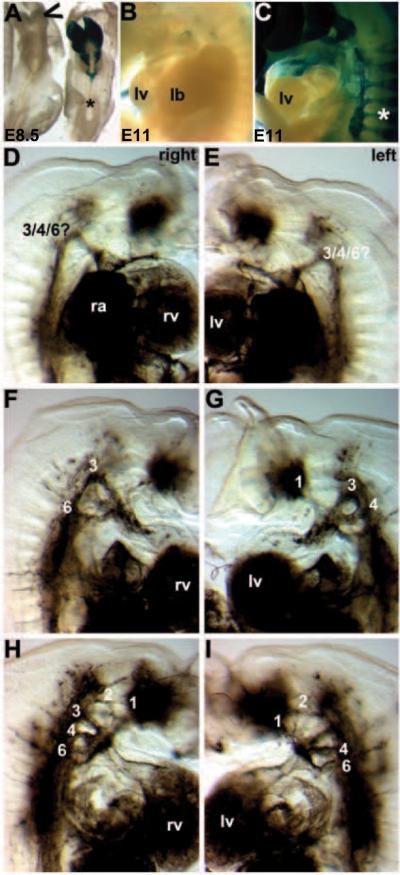
Figure 8. Pathogenesis of Wnt1-Cre;R26-EGFP-DTA-mediated genetic NC ablation cardiovascular defects.
(A) In order to visualize the DTA expressing cells and to determine how quickly the DTA caused NC apoptosis, we utilized the R26R reporter mice that express lacZ in every cell in which Wnt1-Cre is expressed. By E8.5, the lacZ positive Wnt1-Cre-expressing NC are almost completely ablated within the embryo and only a few Wnt1-Cre cells remain in the Wnt1-Cre;R26-EGFP-DTA/R26R triple transgenic cranial region (indicated via open arrowhead). This demonstrates that this system ablated NC within ~6 hours following onset of Wnt1-Cre-mediated recombination. Note that in the Wnt1-Cre;R26R double transgenic non-ablated control littermate, that cranial and CNC are lacZ positive but that the more posterior trunk NC are yet to be labeled (*). (B,C) Similarly, E11Wnt1-Cre;R26-EGFP-DTA;R26R triple transgenic ablated embryos (B) completely lack any CNC within the pharyngeal arches or any colonization of the mutant OFT, but normal Wnt1-Cre;R26R double transgenic non-ablated control littermates (C) exhibit robust CNC colonization. Also note the absence of Wnt1-Cre-marked NC in the mutant DRG, when compared to normal littermates (indicated by * in C). (D–I) Ink was injected into beating hearts of live E11.5 embryos to visualize the vasculature. By E11.5, the PAAs should have undergone asymmetrical remodeling. Consequently in normal embryos, the right 4th and 6th PAAs regress, and the left 4th and 6th persist to become the aortic artery and pulmonary trunk. Three ink injected Wnt1-Cre;R26-EGFP-DTA;R26R triple transgenic ablated mutants illustrating the various PAA remodeling abnormalities both the left and right side of the mutant embryos. (D,E) Mutant one has just a single abnormal persisting PAA on both sides. (F,G) Mutant two has a 3rd and 6th on the right, but persisting 1st, 3rd, 4th PAA on the left. (H,I) Mutant three has persisting 1st, 2nd, 3rd, 4th and 6th on the right, as well as persisting 1st, 2nd, 4th and 6th on the left but no left 3rd PAA.