Abstract
Background and Objectives
The World Health Organization recommends human immunodeficiency virus (HIV)-positive mothers in resource-poor regions heat-treat expressed breastmilk during periods of increased maternal-to-child transmission risk. Flash-heat, a “low tech” pasteurization method, inactivates HIV, but effects on milk protein bioactivity are unknown. The objectives were to measure flash-heat's effect on antimicrobial properties of lactoferrin, lysozyme, and whole milk and on the digestive resistance of lactoferrin and lysozyme.
Methods
Flash-heated and unheated breastmilk aliquots from HIV-positive mothers in South Africa were “spiked” with Staphylococcus aureus and Escherichia coli and then cultured for 0, 3, and 6 hours. Lysozyme and lactoferrin activities were determined by lysis of Micrococcus luteus cells and inhibition of enteropathogenic E. coli, respectively, measured spectrophotometrically. Percentages of proteins surviving in vitro digestion, lactoferrin and lysozyme activity, and bacteriostatic activity of whole milk in heated versus unheated samples were compared.
Results
There was no difference in rate of growth of E. coli or S. aureus in flash-heated versus unheated whole milk (p = 0.61 and p = 0.96, respectively). Mean (95% confidence interval) antibacterial activity of lactoferrin was diminished 11.1% (7.8%, 14.3%) and that of lysozyme by up to 56.6% (47.1%, 64.5%) by flash-heat. Digestion of lysozyme was unaffected (p = 0.12), but 25.4% less lactoferrin survived digestion (p < 0.0001).
Conclusions
In summary, flash-heat resulted in minimally decreased lactoferrin and moderately decreased lysozyme bioactivity, but bacteriostatic activity of whole milk against representative bacteria was unaffected. This suggests flash-heated breastmilk likely has a similar profile of resistance to bacterial contamination as that of unheated milk. Clinical significance of the decreased bioactivity should be tested in clinical trials.
Introduction
The estimated 15.4 million human immunodeficiency virus (HIV)-positive women living in resource-poor settings must balance opposing risks as they decide how to feed their infants.1 Breastmilk can transmit HIV, yet infants who do not receive breastmilk are at much greater risk of malnutrition, serious infections, and mortality.2–9 Accordingly, the World Health Organization (WHO) revised recommendations for HIV-positive mothers in resource-poor regions to exclusively breastfeed for 6 months and then to continue breastfeeding for up to a year with appropriate complementary feeding. Concomitant administration of antiretroviral prophylaxis to the mother or infant during breastfeeding is recommended to decrease maternal-to-child HIV transmission.10 The WHO also recommends home pasteurization of breastmilk for these mothers as an “interim” strategy, e.g., if antiretroviral therapies are temporarily unavailable or the mother or infant is too ill to breastfeed.10
We have previously described a “low tech” method of pasteurization—flash-heat—appropriate for home use in this setting and documented that the method inactivates cell-free11 and cell-associated12 HIV, rids the milk of bacterial contamination,13 and preserves the vast majority of most vitamins14 and immunoglobulins.15 Flash-heat mimics commercial high-temperature, short-time (HTST) pasteurization, the effects of which on human milk have not been extensively studied. Furthermore, flash-heat raises and lowers the milk's temperature more slowly than does its “high tech” counterpart, which rapidly heats liquid to 72°C for 15 seconds, potentially causing greater damage to milk quality. To better understand the “shelf-life” of human milk pasteurized by this method and its capacity to protect the infant against infections, we undertook the current study to evaluate the effects of flash-heat on the antimicrobial properties of whole human milk and the bactericidal and bacteriostatic activities of lysozyme and lactoferrin.
Materials and Methods
Sample source and flash-heat treatment
Fifty breastmilk samples were collected from HIV-infected women in Durban, South Africa between October and December 2004. Clinical and demographic characteristics of these women and breastmilk collection procedures have been previously described.14 In brief, mean (SD [range]) maternal age was 25.9 (4.9 [19–40]) years, body mass index was 27.5 (4.3 [20.0–37.5]) kg/m2, and CD4+ cell count was 527 (255 [27–1,173]); mean infant age was 15 (11 [6–68]) weeks.
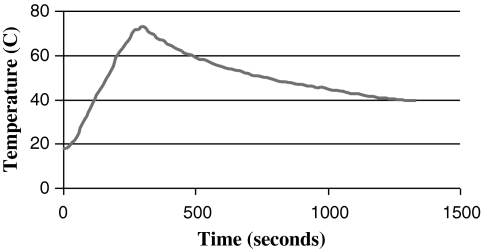
Each sample of fresh milk was divided into two aliquots, one of which was flash-heated in the laboratory under conditions designed to mimic those in the field. In brief, 50 mL of milk was placed in an uncovered 16-oz (455-mL) glass food jar that was then placed in 450 mL of water in a 1:1 Hart brand 1-quart aluminum pan. The water and milk were heated together over a butane stove burner, used to imitate the intense heat of a fire, until the water reached 100°C and was at a rolling boil. The jar of breastmilk was then immediately removed from the water bath and allowed to cool. The milk typically reached a peak temperature of 72.9°C and was above 56.0°C for 6 minutes 15 seconds, the lowest temperature used in low-temperature, long-time pasteurization methods.16 A typical time–temperature curve for the breastmilk is shown in Figure 1. Samples were stored at −70°C until analysis.
FIG. 1.
Typical time–temperature curve of flash-heated breastmilk.
Bacteriostatic activity of whole milk
The effect of heat treatment on the bacteriostatic activity of whole milk was assessed by quantitative subculture at the California Department of Public Health (Richmond, CA). Twenty-one of 50 milk samples were randomly chosen to test for inhibition of bacterial growth. Heated and unheated samples were spiked with Staphylococcus aureus ssp. Rosenbach (catalog number ATCC 25923, American Type Culture Collection, Manassas, VA) or Escherichia coli enteropathogenic serovar O55:K59 (B5) (catalog number ATCC 12014, American Type Culture Collection) as challenge organisms at 1 × 106 colony-forming units (CFU)/mL, diluted in Dulbecco's phosphate-buffered saline (Gibco, Grand Island, NY), incubated at 25°C, and then assayed using quantitative subcultures at 0-, 3-, and 6-hour periods. After the specific incubation period, a 10-μL loop of sample was streaked in triplicate on selective agar: eosin methylene blue for E. coli and mannitol salt agar for S. aureus (both from Hardy Diagnostics, Santa Monica, CA). Colonies were counted on a Quebec counter. Controls (Dulbecco's phosphate-buffered saline, stock cultures, and unspiked experimental milk) were also streaked on appropriate media to check for bacterial contamination, bacterial viability, and background level of bacteria in unspiked milk.
Lysozyme antimicrobial activity
Assessment of the bioactivity of lysozyme in 50 heated versus unheated breastmilk samples was determined by measuring its lysis of Micrococcus luteus cells, calibrated against a lysozyme standard. M. luteus (catalog number ATCC 4698, American Type Culture Collection) was grown for 6 hours at 25°C in trypticase soy broth (Becton Dickinson, Franklin Lakes, NJ). The culture was centrifuged at 800 g, and the pellet was resuspended in potassium phosphate buffer (PPB) (66 mM KH2PO4, pH 6.4) to an optical density (OD) at 450 nm (OD450) of 0.6. Defatted milk was obtained by centrifuging whole milk samples at 5,000 g for 20 minutes. The defatted milk was stored at −80°C until use, and pelleted cells were discarded. The antimicrobial activity of lysozyme in human milk using M. luteus was examined as previously described17 with noted modifications. A 96-well plate (Nunc/Nalgene®, Thermo Scientific, Rochester, NY) was used, and wells were loaded with 250 μL of M. luteus in BBL™ Trypticase™ soy broth (Becton Dickinson), 10 μL of whey diluted 1:4 in PPB, and 10 μL of PPB as a negative control. Purified lysozyme from human milk (Sigma-Aldrich, St. Louis, MO) was used as a positive control. All samples and controls were assayed in quadruplicate. The plate was incubated at 25°C in an enzyme-linked immunosorbent assay reader (Versamax, Molecular Devices Corp., Sunnyvale, CA). The OD450 was recorded every minute for a total of 10 minutes. Slopes of the curves were calculated using linear regression and compared using the signed-rank test.
Lactoferrin antimicrobial activity
Bioactivity of lactoferrin in 29 heated versus unheated breastmilk samples was assessed by measuring its antimicrobial activity against enteropathogenic E. coli serotype O111 (catalog number ATCC 33780, American Type Culture Collection) compared to a commercial lactoferrin standard (Sigma-Aldrich). Lactoferrin was purified from milk whey samples by chromatography using a heparin-Sepharose affinity column (GE Healthcare, Waukesha, WI) as previously described.18 In each flat-bottomed well of a 96-well plate (Corning® [Corning, NY] Costar®), 220 μL of a preparation of enteropathogenic E. coli serotype O111 at 3 × 103 CFU/mL in Difco™ (Detroit, MI) Nutrient Broth 234000 broth and 50 μL of lactoferrin with a final concentration of 300 μg/mL in 0.05 M Tris HCl–1 M NaCl (pH 8) were placed. Each sample was assayed in triplicate. The plate with lid was incubated at 37°C in an enzyme-linked immunosorbent assay reader (Versamax) that performed kinetic readings of OD at 630 nm (OD630) every hour for 20 hours total.
Resistance of lactoferrin and lysozyme to digestion
In vitro digestion was performed on 20 breastmilk samples as previously described with minor modifications.8,19 Milk samples were adjusted to pH 3.8 with 1 M HCl, and porcine pepsin was added to a pepsin/protein ratio of 0.08. After incubation for 30 minutes (37°C and shaking at 200 rpm), pH was adjusted to 7.0 with 1 M NaHCO3, porcine pancreatin was added to a pancreatin/initial protein ratio of 0.016 and incubated for an additional 1 hour. Digestive enzymes were then inactivated by heating to 85°C for 3 minutes. To identify remaining proteins, samples were prepared under reducing conditions by 1:1 dilution with Laemmli sample buffer (Bio-Rad, Hercules, CA) containing 5% 2-mercaptoethanol and heated to 100°C for 5 minutes. Equal amounts of protein (2.5 μg) were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, using 10% and 15% acrylamide for lactoferrin and lysozyme, respectively. Protein was transferred to nitrocellulose membrane for 60 minutes at 350 mA and then washed in phosphate-buffered saline containing 0.1% Tween 20 (PBST) for 3–5 minutes. To minimize nonspecific binding of antibodies, membranes were blocked for 1 hour in PBST containing 5% bovine serum albumin at room temperature with gentle rocking. Blots for lactoferrin were incubated with 1:20,000 diluted anti-human lactoferrin horseradish peroxidase–conjugated antibody (Biodesign International, Saco, ME) in PBST containing 1% bovine serum albumin for 60 minutes at room temperature with gentle rocking. Blots for lysozyme were incubated with 1:7,500 diluted polyclonal rabbit anti-human lysozyme (DakoCytomation, Glostrup, Denmark) in PBST containing 1% bovine serum albumin for 1 hour, washed with PBST, and then incubated with 1:20,000 diluted enhanced chemiluminescence donkey anti-rabbit immunoglobulin G horseradish peroxidase conjugate (Amersham Biosciences, Piscataway, NJ) for 1 hour at room temperature with gentle rocking. After antibody incubation, blots were washed gently with PBST. Bands were detected with enhanced chemiluminescence western blotting detection reagent (Amersham), exposed to film for 10–20 seconds, and quantified using the Chemi-doc Gel Quantification System (Bio-Rad).
Statistical analysis
Statistical analysis was performed with SAS for Windows release 9.1.3 (SAS Institute, Cary, NC). Bacterial growth was logarithm-transformed and compared with an analysis of variance model that included main effects of flash-heating and time, the flash-heating × time interaction, and a random effect of subject. Lysozyme density and lactoferrin density were logarithm-transformed and compared with analysis of variance (SAS Generalized Linear Model procedure), which included main effects of flash-heating (yes/no) and digestion (yes/no), the interaction between flash-heating and digestion, and a random effect of subject. The Tukey–Kramer adjustment was used for multiple comparisons. Slopes of the curves demonstrating lysozyme bioactivity (OD450 vs. time) were calculated using linear regression and compared using the signed-rank test. For all analyses, p < 0.05 was considered statistically significant.
Results
Bacteriostatic activity of whole milk
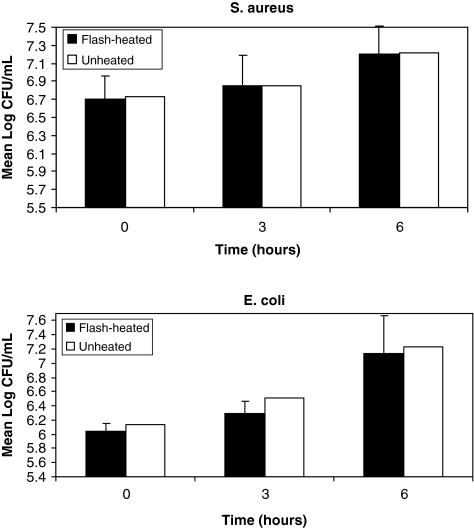
Mean (SD) base-10 logarithm S. aureus concentrations (CFU/mL) at 0, 3, and 6 hours in the heated milk were 6.70 (0.27), 6.85 (0.31), and 7.20(0.34) in the heated milk and 6.72 (0.34), 6.85 (0.36), and 7.21 (0.28) in the unheated milk (Fig. 2). Corresponding concentrations for E. coli were 6.04 (0.11), 6.29 (0.17), and 7.14 (0.52) in the heated milk and 6.13 (0.47), 6.52 (0.64), and 7.23 (0.35) in the unheated milk. There was significant bacterial growth over time, but there was no significant difference in growth rate between the heated and unheated milk samples (p = 0.96 for S. aureus and p = 0.61 for E. coli).
FIG. 2.
Mean S. aureus and E. coli (log colony-forming units [CFU]/mL) growth over 6 hours in flash-heated and unheated breastmilk. No significant difference in growth rates occurred (p = 0.96 and p = 0.61, respectively). Bars represent SDs.
Lysozyme bactericidal activity
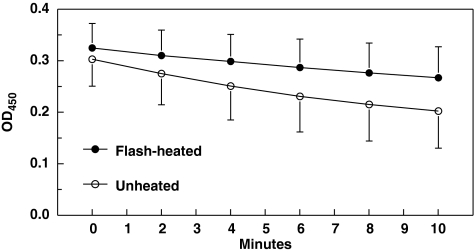
Mean OD450 at all time points are shown in Figure 3. Because of an apparent biphasic decline in M. luteus concentration, mean slopes over the first 4 minutes as well as the entire 10 minutes were both calculated. Over the first 4 minutes, mean (SD) slopes of the curve in flash-heated and unheated milk, respectively, were −0.0225 (0.0161) and −0.0532 (0.0377). Corresponding slopes over 10 minutes were −0.0214 (0.0148) and −0.0459 (0.0249), respectively. Over both time frames, the slope of OD450 versus time was significantly flattened (less negative) in the flash-heated compared with the unheated breastmilk (p < 0.0001), indicating retention of 43.4% (35.5%, 52.9%) and 44.8% (36.9%, 54.4%) of the lysozyme activity in the flash-heated compared to the unheated milk over the two time frames.
FIG. 3.
Slope of optical density at 450 nm (OD450) of M. luteus activity in purified lysozyme over time with flash-heated and unheated breastmilk. Signed-rank test, flash-heated slope different from unheated slope, p < 0.0001.
Lactoferrin bacteriostatic activity
The mean (SD) OD630 at 20 hours of the enteropathogenic E. coli serotype O111 incubated with lactoferrin from flash-heated milk samples was 0.398 (0.064), and with lactoferrin from unheated samples it was 0.358 (0.056), corresponding to mean (SD) log (OD630) for heated samples of −0.406 (0.068) and for unheated samples of −0.451 (0.065) (p < 0.0001). This represents 11.1% (95% confidence interval 7.8%, 14.3%) greater growth of enteropathogenic E. coli serotype O111 when incubated with lactoferrin purified from the flash-heated samples relative to the unheated samples.
Resistance of lactoferrin and lysozyme to digestion
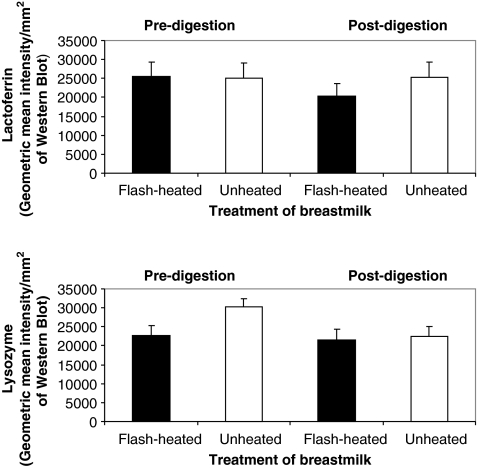
Lactoferrin and lysozyme concentrations pre- and post-digestion in both heated and unheated samples are shown in Figure 4. Lactoferrin concentrations did not significantly differ in pre- versus post-heated undigested breastmilk samples (p = 0.91) Post-digestion concentrations of lactoferrin did show a significant decrease in flash-heated breastmilk whereby 79.8% (75.8%, 84.1%) of heated lactoferrin survived digestion compared to all of the lactoferrin in the unheated breastmilk (p < 0.0001).
FIG. 4.
Lactoferrin and lysozyme concentrations (measured as intensity/mm2 with western blot assay) in unheated and flash-heated breastmilk samples before and after digestion (n = 20).
Lysozyme concentrations, however, did show a significant decrease in undigested flash-heated breastmilk samples to 74.6% (71.3%, 78.0%) of that found in the unheated milk (p < 0.0001) but did not show a significant difference between pre- and post-heated digested breastmilk samples (p = 0.12), indicating that the amount of lysozyme that survives digestion is not affected by heating.
Discussion
There are several results in this study that support the overall safety of flash-heated breastmilk. Perhaps foremost, the bacteriostatic activity of the flash-heated milk is comparable to the unheated breastmilk when contaminants are introduced post-heat. Equivalent antibacterial activity of the heated milk suggests that storage of heated milk at 25°C would be as safe from a bacteriologic standpoint as storage of unheated milk, at least over the 6-hour time frame studied. We strove to make our test system representative of the typical home environment of South African women. The two bacteria chosen for spiking are among the most common pathogens known to circulate in South Africa,20,21 and the incubation temperature of 25°C is representative of the ambient temperature in South Africa.22 To our knowledge, only one other study has evaluated the effect of pasteurization on the antibacterial properties of whole human milk. In contrast to our data showing little impact from flash-heat, they found that other pasteurization methods—heating to 63°C for 30 minutes and 75°C for 15 seconds—have shown a decrease in bactericidal activity over 2 hours against E. coli of 25% and 48%, respectively.23 This difference could be related to less damage by the flash-heat method of pasteurization we used, which required a lower temperature or shorter heating duration than the methods previously tested. Despite our reassuring results on the retention of antibacterial activity in flash-heated breastmilk, safeguards should be put in place to store milk in a manner to minimize bacterial contamination, e.g., storing in the same container as heating and covering the milk with a clean lid.
In addition to equivalent bacteriostatic activity of the whole flash-heated milk, the bioactivity of lactoferrin isolated from the flash-heated breastmilk demonstrated only a minimal decrease of 11.1% in bacteriostatic activity against enteropathogenic E. coli. This is very reassuring, particularly in concert with the fact that there was no decrease in lactoferrin quantity in the flash-heated milk as measured by western blotting in the predigested sample. Preservation of lactoferrin bioactivity has been noted in multiple previous reports with commercial HTST at 72°C for 15–20 seconds.24,25 Minimal impact of the flash-heat method on lactoferrin bioactivity documents that the “low-tech” method, which raises and lowers the temperature of the milk more slowly than commercial methods, does not damage the quality of the milk any more than do commercial HTST methods. We previously reported similar results when comparing the effect of flash-heat and commercial HTST pasteurization on breastmilk immunoglobulins.15
Lysozyme bactericidal activity against M. luteus was more affected by the flash-heat than the bacteriostatic activity of whole milk or lactoferrin. The degree of retained bioactivity, at approximately 45%, does compare favorably, however, to the 39% of retained bioactivity resulting from Holder pasteurization, typically used in human milk banking, reported by Czank et al.26 and the 15–35% reported by Koenig et al.27 As the authors of the latter study noted, the lysozyme activity in the heated milk is still greater than that found in breastmilk substitutes, which are of non-human origin and also pasteurized.
Many human milk proteins with bioactive properties have been shown to be resistant to digestive proteolysis and consequently are able to provide antimicrobial and immunomodulatory functions in the distal gastrointestinal tract.28 Ascertaining the degree to which pasteurization alters resistance to digestion in vitro provides an additional measure of the anticipated clinical effect of consuming heated versus unheated milk. It is reassuring that the flash-heat method had no significant impact on the amount of lysozyme and a relatively minor impact on the amount of lactoferrin surviving digestion, decreasing by only 20% the amount available to provide intraluminal activity in the distal intestine.
There are some limitations to the current study. This study was not designed to ascertain length of safe storage at different room temperatures. Of note is that it is currently recommended that expressed human milk may be stored for 6–8 hours at room temperatures up to 25°C, which was the storage temperature used in the current study.29 There is conflicting evidence regarding storage safety at higher temperatures,30,31 with the result that some authors do not recommend storage at 38°C,30,31 whereas others cite evidence that storage at this temperature is safe for 4 hours30 or even 8 hours.32 We note that equivalent bacteriostatic activity of flash-heated and unheated breastmilk at 25°C suggests that bacteriostatic activity of flash-heated milk would likely be equivalent to that of unheated milk also at higher temperatures, e.g., 38°C, and concur that more studies to document length of safe storage of human milk at these temperatures, heated or unheated, would be helpful. Also, the post-digestion results are to be interpreted with caution. Halting the digestion procedure requires heating the breastmilk samples and may have therefore itself affected the bioactivity in a similar manner to the flash-heat, thereby interfering with our ability to isolate the impact from the flash-heat treatment.
Conclusions
In summary, it is reassuring that the bacteriostatic activity of the flash-heated whole milk was unaffected and that lactoferrin's bioactivity and ability to survive digestion were both minimally impacted. The clinical significance of the observed decrease in bioactivity, most notably of lysozyme, will need further evaluation in prospective clinical trials. These results overall suggest that flash-heat may be a safe method for home pasteurization for HIV-infected mothers in developing countries, of particular value during times of greater risk for maternal-to-child transmission of HIV, such as during episodes of infant oral thrush, maternal mastitis, or interrupted antiretroviral prophylaxis and/or upon addition of complementary foods.
Acknowledgments
The authors would like to acknowledge the mothers who participated in this study and the Cato Manor Clinic staff for their time and dedication. We thank Lindiwe Sibeko for assistance with subject recruitment, Derek Lee for technical assistance with whole milk bacteriostatic experiments, and Andrew Hall for technical assistance with in vitro digestion and western blot assays. Funding for this project was provided by the National Institute of Child Health and Human Development (grant HD051473-01), the University of California, Davis Children's Miracle Network, the Thrasher Research Fund, and the James B. Pendleton Charitable Trust.
Disclosure Statement
No competing financial interests exist.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO) AIDS Epidemic Update. UNAIDS; Geneva: 2009. [Jun 9;2010 ]. [Google Scholar]
- 2.Coutsoudis A. Pillay K. Kuhn L, et al. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: Prospective cohort study from Durban, South Africa. AIDS. 2001;15:379–387. doi: 10.1097/00002030-200102160-00011. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn L. Aldrovandi GM. Sinkala M, et al. Effects of early, abrupt weaning for HIV-free survival of children in Zambia. N Engl J Med. 2008;359:130–141. doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mbori-Ngacha D. Nduati R. John G, et al. Morbidity and mortality in breastfed and formula-fed infants of HIV-1-infected women: A randomized clinical trial. JAMA. 2001;286:2413–2420. doi: 10.1001/jama.286.19.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becquet R. Bequet L. Ekouevi DK, et al. Two-year morbidity-mortality and alternatives to prolonged breast-feeding among children born to HIV-infected mothers in Cote d'Ivoire. PLoS Med. 2007;4:e17. doi: 10.1371/journal.pmed.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becquet R. Leroy V. Ekouevi DK, et al. Complementary feeding adequacy in relation to nutritional status among early weaned breastfed children who are born to HIV-infected mothers: ANRS 1201/1202 Ditrame Plus, Abidjan, Cote d'Ivoire. Pediatrics. 2006;117:e701–e710. doi: 10.1542/peds.2005-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson W. Alons C. Fidalgo L, et al. The challenge of providing adequate infant nutrition following early breastfeeding cessation by HIV-positive, food-insecure Mozambican mothers; Presented at the XVI International AIDS Conference; Toronto, Canada. 2006. [Google Scholar]
- 8.Creek TL. Kim A. Lu L, et al. Hospitalization and mortality among primarily nonbreastfed children during a large outbreak of diarrhea and malnutrition in Botswana, 2006. J Acquir Immune Defic Syndr. 2010;53:14–19. doi: 10.1097/QAI.0b013e3181bdf676. [DOI] [PubMed] [Google Scholar]
- 9.Kourtis AP. Fitzgerald G. Hyde L, et al. Diarrhea in uninfected infants of HIV-infected mothers who stop breastfeeding at 6 months: The BAN study experience; Presented at the XIV Conference on Retroviruses and Opportunistic Infections; Los Angeles. 2007. [Google Scholar]
- 10.World Health Organization. Rapid Advice. World Health Organization; Geneva: 2009. [Jun 9;2010 ]. HIV and Infant Feeding: Revised Principles and Recommendations. [Google Scholar]
- 11.Israel-Ballard K. Donovan R. Chantry C, et al. Flash-heat inactivation of HIV-1 in human milk: A potential method to reduce postnatal transmission in developing countries. J Acquir Immune Defic Syndr. 2007;45:318–323. doi: 10.1097/QAI.0b013e318074eeca. [DOI] [PubMed] [Google Scholar]
- 12.Volk ML. Hanson CV. Israel-Ballard K, et al. Inactivation of cell-associated and cell-free HIV-1 by flash-heat treatment of breast milk. J Acquir Immune Defic Syndr. 2010;53:665–666. doi: 10.1097/QAI.0b013e3181ba47df. [DOI] [PubMed] [Google Scholar]
- 13.Israel-Ballard K. Coutsoudis A. Chantry CJ, et al. Bacterial safety of flash-heated and unheated expressed breastmilk during storage. J Trop Pediatr. 2006;52:399–405. doi: 10.1093/tropej/fml043. [DOI] [PubMed] [Google Scholar]
- 14.Israel-Ballard K. Abrams B. Coutsoudis A, et al. Vitamin content of breastmilk from HIV-1 infected mothers before and after flash-heat treatment. J Acquir Immune Defic Syndr. 2008;48:444–449. doi: 10.1097/QAI.0b013e31817beb8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chantry CJ. Israel-Ballard K. Moldoveanu Z, et al. Effect of flash-heat treatment on immunoglobulins in breast milk. J Acquir Immune Defic Syndr. 2009;51:264–267. doi: 10.1097/QAI.0b013e3181aa12f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence RA. Milk banking: The influence of storage procedures and subsequent processing on immunologic components of human milk. Adv Nutr Res. 2001;10:389–404. doi: 10.1007/978-1-4615-0661-4_19. [DOI] [PubMed] [Google Scholar]
- 17.Huang J. Wu L. Yalda D, et al. Expression of functional recombinant human lysozyme in transgenic rice cell culture. Transgenic Res. 2002;11:229–239. doi: 10.1023/a:1015663706259. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki YA. Kelleher SL. Yalda D, et al. Expression, characterization, and biological activity of recombinant human lactoferrin in rice. J Pediatr Gastroenterol Nutr. 2003;36:190–199. doi: 10.1097/00005176-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Lönnerdal B. Glazier C. An approach to assessing trace element bioavailability from milk in vitro. Extrinsic labeling and proteolytic degradation. Biol Trace Elem Res. 1989;19:57–69. doi: 10.1007/BF02925449. [DOI] [PubMed] [Google Scholar]
- 20.Galane PM. Le Roux ML. Molecular epidemiology of Escherichia coli isolated from young South African children with diarrhoeal diseases. J Health Popul Nutr. 2001;19:31–38. [PubMed] [Google Scholar]
- 21.Campbell SJ. Deshumukh HS. Nelson CL, et al. Genotypic characteristics of Staphylococcus aureus isolates from a multinational trial of complicated skin and skin structure infections. J Clin Microbiol. 2008;46:678–684. doi: 10.1128/JCM.01822-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.About.com: Africa Travel. South Africa's Weather and Average Temperatures. goafrica.about.com/library/bl.weather.southafrica.htm#anchor4. [Jul 17;2010 ]. goafrica.about.com/library/bl.weather.southafrica.htm#anchor4
- 23.Silvestre D. Ruiz P. Martínez-Costa C, et al. Effect of pasteurization on the bactericidal capacity of human milk. J Hum Lact. 2008;24:371–376. doi: 10.1177/0890334408319158. [DOI] [PubMed] [Google Scholar]
- 24.Conesa C. Rota C. Castillo E, et al. Antibacterial activity of recombinant human lactoferrin from rice: Effect of heat treatment. Biosci Biotechnol Biochem. 2009;73:1301–1307. doi: 10.1271/bbb.80814. [DOI] [PubMed] [Google Scholar]
- 25.Goldblum RM. Dill CW. Albrecht TB, et al. Rapid high-temperature treatment of human milk. J Pediatr. 1984;104:380–385. doi: 10.1016/s0022-3476(84)81099-9. [DOI] [PubMed] [Google Scholar]
- 26.Czank C. Prime DK. Hartmann B, et al. Retention of the immunological proteins of pasteurized human milk in relation to pasteurizer design and practice. Pediatr Res. 2009;66:374–379. doi: 10.1203/PDR.0b013e3181b4554a. [DOI] [PubMed] [Google Scholar]
- 27.Koenig A. de Albuquerque Diniz EM. Barbosa SF, et al. Immunologic factors in human milk: The effects of gestational age and pasteurization. J Hum Lact. 2005;21:439–443. doi: 10.1177/0890334405280652. [DOI] [PubMed] [Google Scholar]
- 28.Lönnerdal B. Bioactive proteins in human milk: Mechanisms of action. J Pediatr. 2010;156(2 Suppl):S26–S30. doi: 10.1016/j.jpeds.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 29.The Academy of Breastfeeding Medicine Protocol Committee. Human milk storage information for home for full-term infants 2010 Revision. Breastfeed Med. 2010;5:127–130. doi: 10.1089/bfm.2010.9988. [DOI] [PubMed] [Google Scholar]
- 30.Hamosh M. Ellis LA. Pollock DR, et al. Breastfeeding and the working mother: Effect of time and temperature of short-term storage on proteolysis, lipolysis, and bacterial growth in milk. Pediatrics. 1996;97:492–498. [PubMed] [Google Scholar]
- 31.Igumbor EO. Mukura RD. Makandiramba B, et al. Storage of breast milk: Effect of temperature and storage duration on microbial growth. Cent Afr J Med. 2000;46:247–251. doi: 10.4314/cajm.v46i9.8564. [DOI] [PubMed] [Google Scholar]
- 32.Ajusi JD. Onyango FE. Mutanda LN, et al. Bacteriology of unheated expressed breast milk stored at room temperature. East Afr Med J. 1989;66:381–387. [PubMed] [Google Scholar]