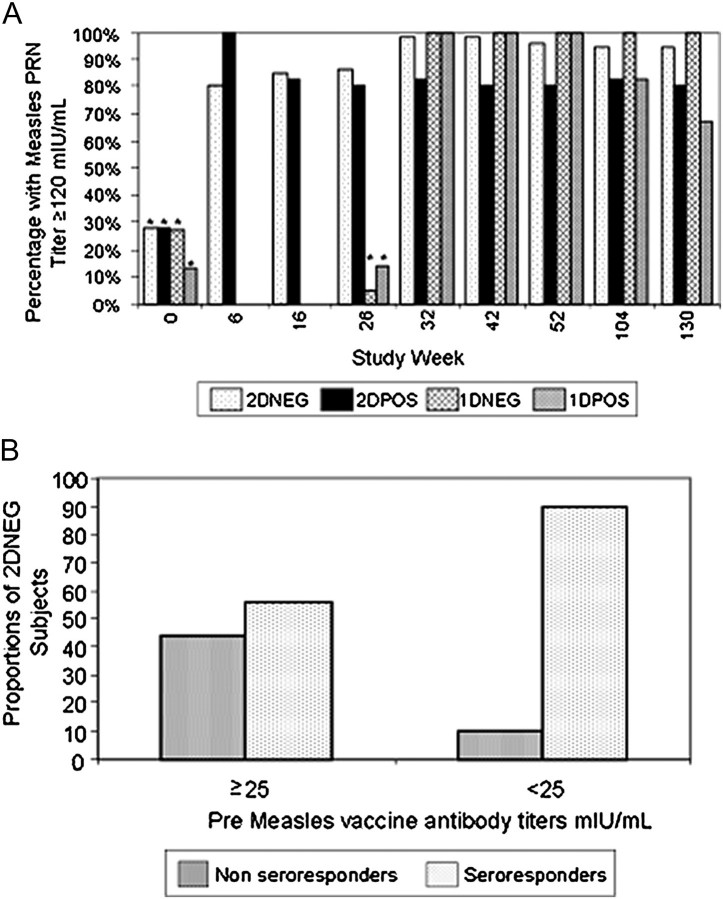
Figure 3.
HIV-infected (POS) and uninfected (NEG) children enrolled in PACTG 225 were randomized to 1 of 2 measles vaccine regimens: 2D children were given monovalent measles vaccine at 6 months of age (study week 0) followed by MMRII vaccine at 12 months of age (study week 26); 1D children were only given MMRII at 12 months of age only. Subjects were followed until ∼3 years of age (study week 130). Measles PRN titers were measured in parallel with the Measles International Reference Serum (WHO 66/202) and are expressed in mIU/mL with a titer of 1:8 equivalent to 8 mIU/mL in this assay. Panel A shows pre- and postvaccination measles PRN titers. Bars marked with an asterisk (*) designate the proportion of study subjects with measles PRN titers ≥25 mIU/mL. Bars not marked with an asterisk represent PRN titers ≥120 mIU/mL at study weeks 6, 16, 26, 32, 42, 52, 104, and 130 by treatment group. Panel B is a further analysis of the study week 6 postdose 1 data for 2DNEG subjects only and shows the percent of seroresponders (PRN titer ≥120 mIU/mL) stratified by prevaccination titer ≥25 mIU/mL or <25 mIU/mL.
PACTG indicates Pediatric AIDS Clinical Trials Group; MMRII, measles, mumps, and rubella, live attenuated; WHO, World Health Organization; PRN, plaque reduction neutralization.