Abstract
BACKGROUND: Rare variants of the WFS1 gene encoding wolframin cause Wolfram syndrome, a monogenic disease associated with diabetes insipidus, diabetes mellitus, optic atrophy, and deafness. In contrast, common variants of WFS1 showed association with type 2 diabetes (T2D) in numerous Caucasian populations. AIM: In this study, we tested whether the markers rs752854, rs10010131, and rs734312, located in the WFS1 gene, are related to the development of T2D in a Russian population. METHODS: The polymorphic markers were genotyped in Russian diabetic (n = 1,112) and non-diabetic (n = 1,097) patients using a Taqman allele discrimination assay. The correlation between the carriage of disease-associated WFS1 variants and the patients' clinical and metabolic characteristics was studied using ANOVA and ANCOVA. Adjustment for confounding variables such as gender, age, body mass index, obesity, HbA1c, and hypertension was made. RESULTS: Haplotype GAG, consisting of the minor alleles of rs752854, rs10010131, and rs734312, respectively, showed association with decreased risk of T2D (OR = 0.44, 95% CI = 0.32-0.61, p = 4.3 x 10-7). Compared to other WFS1 variants, non-diabetic individuals homozygous for GAG/CAG had significantly increased fasting insulin (padjusted = 0.047) and homeostasis model assessment of β-cell function (HOMA-β) index (padjusted = 0.006). Diabetic patients homozygous for GAG/GAG showed significantly elevated levels of 2-h insulin (padjusted = 0.029) and HOMA-β = 0.011. CONCLUSIONS: Disease-associated variants of WFS1 contribute to the pathogenesis of T2D through impaired insulin response to glucose stimulation and altered β-cell function.
Keywords: type 2 diabetes, WFS1, wolframin, polymorphism, endoplasmic reticulum stress, beta-cell function, OGTT, fasting plasma glucose
Abbreviations: ANOVA - analysis of variance; ANCOVA - analysis of covariance; ATF6 - activating transcription factor 6; BMI - body mass index; BP - blood pressure; CI - confidence interval; DBP - diastolic blood pressure; DNA - deoxyribonucleic acid; ER - endoplasmic reticulum; FFA - free fatty acids; FPG - fasting plasma glucose; HbA1c - glycated hemoglobin; HDL - high-density lipoprotein; HDL-C - high-density lipoprotein cholesterol; HOMA-beta - homeostasis model assessment of beta-cell function; HOMA-IR - homeostasis model assessment of insulin resistance; ISI - insulin sensitivity index; LD - linkage; LDL - low-density lipoprotein; LDL-C - low-density lipoprotein cholesterol; Na+/K+-ATPase - sodium-potassium adenosine triphosphatase (also known as sodium-potassium pump); NEFA - non-esterified fatty acids assay; OGTT - oral glucose tolerance test; OR - odds ratio; PG - plasma glucose; SBP - systolic blood pressure; SD - standard deviation; SMAD - homolog of drosophila protein, mothers against decapentaplegic (MAD), and Caenorhabditis elegans protein SMA; Smurf1 - SMAD ubiquitination regulatory factor 1; SNP - single nucleotide polymorphism; STRAP structure-based sequences alignment program; T2D - type 2 diabetes; TG - triglycerides; WFS1 - wolfram syndrome 1 gene; WHO - World Health Organization
Introduction
Loss-of-function mutations in the WFS1 gene encoding wolframin cause Wolfram syndrome (OMIM 222300), which is associated with diabetes insipidus, diabetes mellitus, optic atrophy, and deafness [1]. Wolfram syndrome is a rare autosomal-recessive disorder. Causal variants were mapped by positional cloning on the short arm of chromosome 4 (4p16.1) containing the WFS1 gene [2, 3]. The disease is accompanied with progressive loss of sensory neurons and pancreatic β-cells. Given the impact of WFS1 variants on a monogenic form of diabetes, WFS1 was considered as a likely functional candidate gene for association with a common form of type 2 diabetes (T2D). First evidence for association between WFS1 and T2D was obtained in a small study involving an analysis of UK families with T2D [4]. The association between WFS1 and T2D was then repeatedly replicated by several large-scale studies in various Caucasian populations [5-10]. In WFS1, several single nucleotide polymorphisms (SNPs) showed significant associations with T2D, with SNP rs10010131 as the most strongly disease-associated marker (for a minor allele A, odds ratio (OR) = 0.89, 95% confidence interval (95% CI) = 0.86-0.92) [7]. At present, there are no publicly available reports evaluating whether genetic variants of WFS1 are implicated in the pathogenesis of T2D in Russian patients. However, in one study, an association between SNP rs1801211 and sporadic Parkinson's disease was found in a Russian population [11]. In our study, we examined whether markers rs734312, rs10010131, and 752854, which showed the most robust association in T2D in Europeans, are associated with the development of T2D in a Russian population.
Materials and methods
Patients
We studied a total of 2,209 unrelated patients aged 50 years and older. 1,112 of whom were affected by T2D. The remaining 1,097 control individuals were normoglycemic, and had no clinical diabetes. According to the patients’ questionnaires, 1,944 (88% of 2,209) participants had four grandparents of Russian ancestry, the remaining 265 patients had three grandparents of Russian ancestry, and one of either Ukrainian, or Belarusian descent. A total of 794 (402 affected and 392 non-affected) individuals living in Moscow, and neighboring areas, were recruited by the Endocrinology Research Center. The second cohort (710 diabetic and 705 non-diabetic residents of Tymen) was collected by the Tyumen State Medical Academy. T2D was defined according to WHO diagnostic criteria. Thus, participants were regarded as diabetic if either their fasting plasma glucose concentration was ≥7.8 mmol/l, or their plasma glucose concentration was ≥11.1 mmol/l 2 h after a 75-g oral glucose tolerance test (OGTT) [12]. Another criterion was treatment by glucose-lowering agents. Hypertension was defined as systolic pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg in at least two separate measurements, or a history of hypertension. Obesity was defined as BMI ≥30 kg/m2 [13]. All study participants resided in Moscow, or Moscow region. Controls had no past history of glucose intolerance, a glycated hemoglobin (HbA1c) level of <6.4%, or a normal OGTT, and no family history of diabetes. To avoid interference from biological variables, people with a previous diagnosis of type 1 diabetes, gestational diabetes, rare forms of T2D, secondary diabetes (pancreatitis, hemochromatosis), and hypercholesterolemia, or those undergoing treatment with cholesterol-lowering drugs, were excluded from the study. The study protocol was approved by the Review Board of the Endocrinology Research Center, and all participants provided written informed consent.
Biochemical measurements
Fasting cholesterol, HDL, cholesterol, triglycerides, and plasma glucose were measured by standard enzymatic assays. Fasting plasma FFA concentrations were assayed by an enzymatic colorimetric method using a commercially available kit (WAKO NEFA C-test; Waco Chemicals, Neuss, Germany). LDL cholesterol was derived using the Friedewald equation [14]. A standard 75-g OGTT was performed after a 12-h overnight fast according to WHO recommendations. HbA1c was measured using ion-exchange high performance liquid chromatography (normal reference range: 4.1-6.4%). Plasma insulin levels were determined by means of an enzymatic immunoassay. Homeostasis model assessment of β-cell function (HOMA-β) was calculated as 20 x fasting plasma insulin (mU/l)/(fasting glucose (mmol/l) - 3.5). Homeostasis model assessment of insulin resistance (HOMA-IR) was computed as fasting plasma insulin (mU/l) x fasting glucose (mmol/l)/ 22.5 [15]. Insulin sensitivity index (ISI) was assessed as ISI = exp (2.63 - 0.28 x ln (fasting plasma insulin (mU/L)) - 0.31 x ln (serum triglycerides (mmol/l))) [16]. HOMA-IR ≥ 3.8 and ISI ≤ 6.3 were considered as threshold scores reflecting the presence of IR. Clinical and metabolic characteristics of diabetic and non-diabetic participants are summarized in Table 1.
Table 1. Clinical and metabolic characteristics of the patients.
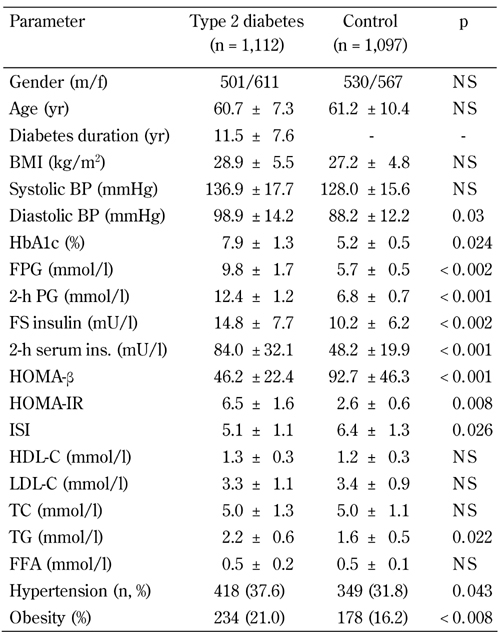
Legend: Data are mean ± SD. Data compared using chi-squared test (gender, hypertension, obesity), or unpaired Student's t-test (other). BMI: body mass index. BP: blood pressure. HbA1c: glycated hemoglobin. FPG: fasting plasma glucose. PG: plasma glucose. FS: fasting serum. HOMA-β: homeostasis model assessment of β-cells. HOMA-IR: homeostasis model assessment of insulin resistance. ISI: insulin sensitivity index. HDL-C: high-density lipoprotein cholesterol. LDL-C: low-density lipoprotein cholesterol. TC: total cholesterol. TG: triglycerides. FFA: free fatty acids. NS: not significant.
DNA analysis
Total DNA was isolated from whole-blood samples pretreated with proteinase K using a standard protocol for extraction with phenol-chloroform. Genotyping of rs734312, rs10010131, and rs752854 in WFS1 was performed by a Taqman-based allelic discrimination on a Real-Time PCR System 7500 (Applied Biosystems, Foster City, CA, USA) using the recommended protocol [17]. Overall, genotype calling rate was 99.0% for rs10010131, 98.8% for rs734312, and 98.1% for rs752854.
Statistical analysis
Data were analyzed with SPSS/Win programs (version 13.0; SPSS Inc., Chicago, IL, USA). Results are given as mean ± SD, or percentages. Skewed variables for the continuous traits were log-transformed before statistical comparisons were made. For comparison of quantitative data in groups of affected and non-affected patients, unpaired Student's t-test was used. Tests for Hardy-Weinberg equilibrium and comparison of genotype and allele frequencies in the T2D subjects and controls were performed using the chi-squared test with Yates' correction. OR and 95% CI were used to assess the extent of association of the various genotypes with T2D. A wild-type genotype was used as a reference group to provide separate OR for each genotype.
Pair-wise linkage disequilibrium (LD) between markers and haplotype estimation was calculated using ARLEQUIN v.2.0 software [18]. The significance of interaction between clinical characteristics and haplogenotypes was assessed by two-way ANOVA. Observed relationships were adjusted for patients' conventional risk factors by ANCOVA using age, gender, HbA1c, BMI, obesity, and hypertension as covariates. P-values of less than 0.05 were considered significant. To align species-specific protein sequences of wolframin, the web-based interactive structure-based sequences alignment program (STRAP) was used (available at http://3d-alignment.eu/).
Results
Observed genotype frequencies of all polymorphic markers of WFS1 were in accordance with the Hardy-Weinberg equilibrium (data not shown). Among three SNPs studied, markers rs10010131 and rs752854 showed significant associations with T2D in the Russian population (Table 2). For SNP rs10010131, a minor allele A was associated with reduced disease risk (OR = 0.77, 95% CI = 0.68-0.87, p = 2.4 x 10-5). Similarly, the minor allele G of rs752854 was also related to decreased risk of T2D (OR = 0.86, 95% CI = 0.75-0.96, p = 0.013). The minor allele G of marker rs752854 showed borderline association with a lower diabetes risk (OR = 0.87, 95% CI = 0.76-0.99, p = 0.05). However, after conditioning on effect of SNP rs10010131, associations between two other markers and the disease became non-significant (for rs752854, OR = 0.98, 95% CI = 0.83-1.14, p = 0.91; for rs734312, OR = 0.95, 95% CI = 0.82-1.1, p = 0.79). Therefore, rs10010131 is the only marker strongly associated with diabetes. The association of two other SNPs is rather secondary and influenced by rs10010131.
Table 2. Association of WFS1 variants with type 2 diabetes.
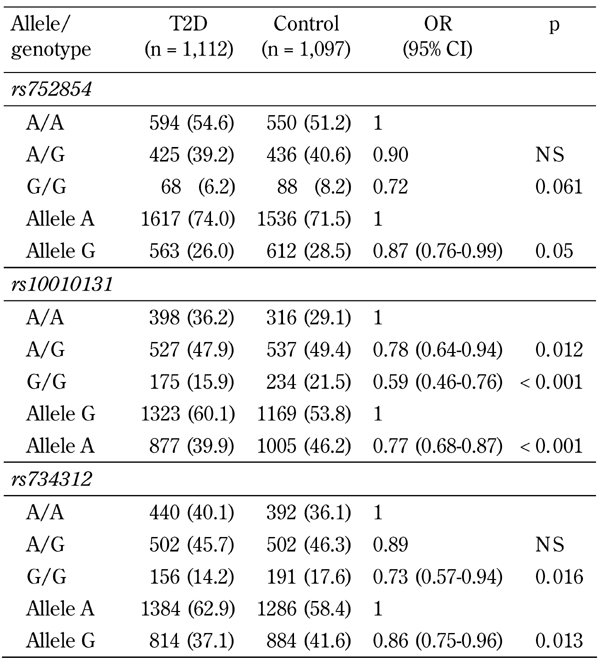
Legend: Data are n (%). A wild-type genotype is used as a reference group (OR =1). OR: odds ratio. CI: confidence interval.
The marker rs752854 was in moderate LD with the SNP rs10010131 (D' = 0.75, p = 0.009), while rs10010131 showed mild but significant LD with the marker rs734312 (D' =0.69, p = 0.039). No significant pair-wise LD were found between rs752854 and 734312 (D' = 0.48, p = 0.39). Major alleles of these markers constituting the most frequent haplotype AGA were significantly associated with a higher diabetes risk (OR = 1.21, 95% CI = 1.06-1.38, p = 0.0056) (Table 3). In contrast, the rarest haplotype GAG consisting of the minor alleles of the markers showed highly significant association with decreased risk of T2D (OR = 0.44, 95% CI = 0.32-0.61, p = 4.3 x 10-7). We studied whether the carriage of disease-associated WFS1 variants is correlated with various metabolic traits associated with metabolic syndrome and T2D. Since the association between markers rs734312 and rs752854 and diabetes is dependent on the effect from rs10010131, we focused on the relation between SNP rs10010131 and metabolic traits. Compared to other WFS1 variants, both diabetic and non-diabetic carriers of the protective genotype AA of rs10010131 had higher HOMA-β, suggesting improved homeostasis of β-cells in these carriers (Table 4). We also considered whether the carriage of WFS1 haplogenotypes is associated with diabetes-related metabolic traits. Each of the groups of diabetic and non-diabetic patients was divided into three subgroups carrying one, two, or no copies of the protective haplogenotype GAG/GAG. After adjustment for confounding variables, we observed significantly increased levels of fasting insulin (padjusted = 0.047) and HOMA-β (padjusted = 0.006) in the controls homozygous for GAG/GAG, compared with the carriers of other WFS1 variants (Table 5). In T2D patients, homozygotes GAG/GAG had significantly elevated 2-h insulin (padjusted = 0.027) and HOMA-β (padjusted = 0.011) as well. These observations suggest that the protective role of the WFS1 haplogenotype GAG/GAG may be related to a higher production of insulin and better function of pancreatic β-cells, as reflected by the HOMA-β value. The SNP rs10010131 itself is associated with improved function of β-cells. However, taking minor alleles of rs734312 and rs752854 into account results in the formation of the protective haplogenotype GAG/GAG, whose association with better β-cell function is even stronger than that of rs10010131 alone.
Table 3. Association of WFS1 haplotypes with type 2 diabetes.
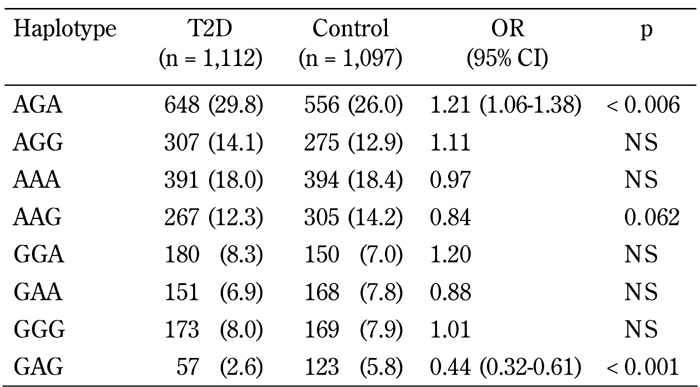
Legend: Data are n (%). Haplotype AGA includes allele A of rs752854, allele G of rs10010131, and allele A of rs734312. OR: odds ratio. CI: confidence interval.
Table 4. Association of SNP rs10010131 of WFS1 with metabolic characteristics of type 2 diabetic patients and non-diabetic controls.
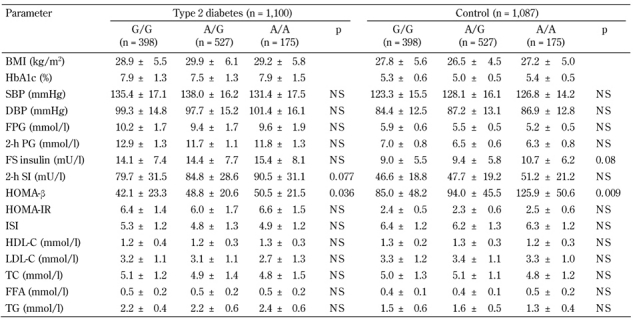
Legend: Data are mean ± SD. P-values adjusted for gender, age, BMI, obesity, HbA1c, and hypertension. BMI: body mass index. HbA1c: glycated hemoglobin. SBP: systolic blood pressure. DBP: diastolic blood pressure. FPG: fasting plasma glucose. PG: plasma glucose. FS: fasting serum. HOMA-β: homeostasis model assessment of β-cells. HOMA-IR: homeostasis model assessment of insulin resistance. ISI: insulin sensitivity index. HDL-C: high-density lipoprotein cholesterol. LDL-C: low-density lipoprotein cholesterol. TC: total cholesterol. TG: triglycerides. FFA: free fatty acids. NS: not significant.
Table 5. Association of haplogenotypes of WFS1 with metabolic characteristics of type 2 diabetic patients and non-diabetic controls.
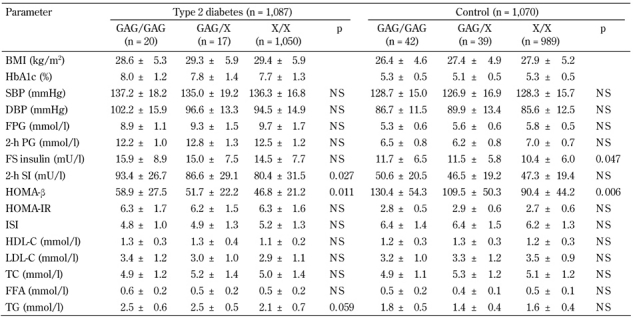
Legend: Data are mean ± SD. P-values adjusted for gender, age, BMI, obesity, HbA1c, and hypertension. Haplotype GAG includes allele G of rs752854, allele A of rs10010131, and allele G of rs734312. X designates any haplotype other than the haplotype GAG. BMI: body mass index. HbA1c: glycated hemoglobin. SBP: systolic blood pressure. DBP: diastolic blood pressure. FPG: fasting plasma glucose. PG: plasma glucose. FS: fasting serum. HOMA-β: homeostasis model assessment of β-cells. HOMA-IR: homeostasis model assessment of insulin resistance. ISI: insulin sensitivity index. HDL-C: high-density lipoprotein cholesterol. LDL-C: low-density lipoprotein cholesterol. TC: total cholesterol. TG: triglycerides. FFA: free fatty acids. NS: not significant.
Discussion
We found associations between WFS1 genetic variants and T2D in Russian patients, thereby confirming results of earlier studies that showed the involvement of WFS1 polymorphisms in conferring susceptibility/resistance to T2D in multiple populations of Caucasians. In our study, diabetic patients homozygous for the protective WFS1 haplogenotype GAG/GAG containing alleles of rs752854, rs1001013, and 734312 showed increased levels of 2-h insulin and HOMA-β. In contrast, carriers of a predisposing WFS1 variant such as the haplogenotype AGA/AGA had reduced serum insulin 2-h post-OGTT and HOMA-β (data not shown). Therefore, carriage of the susceptibility WFS1 variant is related to impaired glucose-induced insulin response resulting from β-cell dysfunction. These results are in accordance with findings in other populations reporting the relationship between the carriage of various disease-associated variants of WFS1 and altered insulin secretion in response to glucose stimulation [10, 19-21] and lower pancreatic β-cell function [22].
SNP rs734312 is a missense mutation that is situated in the last exon (exon 8) of WFS1, and leads to an amino acid (aa) substitution of histidine to arginine in codon 611 (H611R). The H611R polymorphism resides in the cytoplasmic loop between the eighth and ninth transmembrane segments [23]. There are no data reporting whether this SNP is functionally relevant. However, the alignment of the human WFS1 aa sequence against protein sequences of wolframin from other mammals showed that arginine-611 is evolutionary preserved in primates and rodents (Figure 1). Markers rs752854 and rs10010131 are located in non-coding regions (intron 2 and intron 4, respectively) of the WFS1 gene. Therefore, these markers tag the etiological marker of WFS1, which is still unknown.
Figure 1.

Alignment of the protein sequence of human wolframin (aa 599-648) against wolframin aa sequences of other mammals. Arginine (R) at codon 611 is highlighted in gray.
Wolframin is a 100 kDa transmembrane glycoprotein expressed in neural tissues and β-cells. The hypothetical structure of this protein comprising 890 aa includes the N-terminal cytoplasmic domain, nine transmembrane segments (aa 314-652), and a C-terminal tail [23]. Wolframin is incorporated into the endoplasmic reticulum (ER) membrane, and its C-terminus is exposed to the ER lumen. Using a yeast two-hybrid system, the WFS1 C-terminal and transmembrane domains were shown to interact with Na+/K+-ATPase β1 subunit [24]. Wfs1-deficient mice exhibit typical phenotypic outcomes of diabetes, such as progressive glucose intolerance and concomitant insulin deficiency, as a consequence of reduced β-cell mass resulting from impaired cell cycle progression, increased apoptosis, and ER stress [25-27].
The proposed functions of wolframin include the regulation of membrane trafficking, protein processing and calcium homeostasis in the ER of neurons and pancreatic β-cells [28, 29]. This protein negatively regulates ER stress by suppressing the expression of activating transcription factor 6α (ATF6α)-dependent target genes, stabilizing the E3 ubiquitin ligase HRD1, and enhancing ubiquitination and proteasome-mediated degradation of ATF6α [30]. In β-cells, wolframin was recently found to be involved in insulin secretion through intragranular acidification that is necessary for the priming of secretory granules preceding exocytosis [31].
In both diabetes and Wolfram syndrome, WFS1 expression in β-cells is reduced [32]. Furthermore, in β-cells with impaired intracellular signaling, wolframin may become the subject of enhanced ubiquitination and proteasomal degradation mediated by E3 ligase Smurf1 [33]. Therefore, upon WFS1 deficiency, accumulation of misfolded and unfolded proteins result in ER stress that may lead to increased apoptotic β-cell death. Also, insulin processing and secretion may be impaired in diabetes, and may result in increased circulating proinsulin levels due to the disturbance in wolframin-dependent acidification of secretory granules [31].
Further studies are required to identify an etiological marker in WFS1. A recent comprehensive fine-mapping analysis of the WFS1 gene performed in UK Caucasians with the consideration of rare variants (minor allele frequency < 0.01) revealed several putative causal variants for T2D association [9]. However, it was impossible to distinguish between their effects on disease risk due to strong LD between the SNPs within the candidate interval. To distinguish between their effects, it is necessary to study very large population datasets with size exceeding 100,000 records.
Disclosures (conflict of interests statement): The authors report no conflict of interests.
Acknowledgments
The work was supported by grant no. 10-04-01496-а from the Russian Foundation for Basic Research.
References
- 1.Rigoli L, Lombardo F, Di Bella C. Wolfram syndrome and WFS1 gene. Clin Genet. 2011;79:103–117. doi: 10.1111/j.1399-0004.2010.01522.x. [DOI] [PubMed] [Google Scholar]
- 2.Polymeropoulos MH, Swift RG, Swift M. Linkage of the gene for Wolfram syndrome to markers on the short arm of chromosome 4. Nat Genet. 1994;8:95–97. doi: 10.1038/ng0994-95. [DOI] [PubMed] [Google Scholar]
- 3.Inoue H, Tanizawa Y, Wasson J, Behn P, Kalidas K, Bernal-Mizrachi E, Mueckler M, Marshall H, Donis-Keller H, Rogers D. et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome) Nat Genet. 1998;20:143–148. doi: 10.1038/2441. [DOI] [PubMed] [Google Scholar]
- 4.Minton JA, Hattersley AT, Owen K, McCarthy MI, Walker M, Latif F, Barrett T, Frayling TM. Association studies of genetic variation in the WFS1 gene and type 2 diabetes in U.K. populations. Diabetes. 2002;51:1287–1290. doi: 10.2337/diabetes.51.4.1287. [DOI] [PubMed] [Google Scholar]
- 5.Sandhu MS, Weedon MN, Fawcett KA, Wasson J, Debenham SL, Daly A, Lango H, Frayling TM, Neumann RJ, Sherva R. et al. Common variants in WFS1 confer risk of type 2 diabetes. Nat Genet. 2007;39:951–953. doi: 10.1038/ng2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Florez JC, Jablonski KA, McAteer J, Sandhu MS, Wareham NJ, Barroso I, Franks PW, Altshuler D, Knowler WC. Testing of diabetes-associated WFS1 polymorphisms in the Diabetes Prevention Program. Diabetologia. 2008;51:451–457. doi: 10.1007/s00125-007-0891-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franks PW, Rolandsson O, Debenham SL, Fawcett KA, Payne F, Dina C, Froguel P, Mohlke KL, Willer C, Olsson T. et al. Replication of the association between variants in WFS1 and risk of type 2 diabetes in European populations. Diabetologia. 2008;51:458–463. doi: 10.1007/s00125-007-0887-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasson J, Permutt MA. Candidate gene studies reveal that the WFS1 gene joins the expanding list of novel type 2 diabetes genes. Diabetologia. 2008;51:391–393. doi: 10.1007/s00125-007-0920-9. [DOI] [PubMed] [Google Scholar]
- 9.Fawcett KA, Wheeler E, Morris AP, Ricketts SL, Hallmans G, Rolandsson O, Daly A, Wasson J, Permutt A, Hattersley AT. et al. Detailed investigation of the role of common and low-frequency WFS1 variants in type 2 diabetes risk. Diabetes. 2010;59:741–746. doi: 10.2337/db09-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheurfa N, Brenner GM, Reis AF, Dubois-Laforgue D, Roussel R, Tichet J, Lantieri O, Balkau B, Fumeron F, Timsit J. et al. Decreased insulin secretion and increased risk of type 2 diabetes associated with allelic variations of the WFS1 gene: the Data from Epidemiological Study on the Insulin Resistance Syndrome (DESIR) prospective study. Diabetologia. 2011;54:554–562. doi: 10.1007/s00125-010-1989-0. [DOI] [PubMed] [Google Scholar]
- 11.Shadrina M, Nikopensius T, Slominsky P, Illarioshkin S, Bagyeva G, Markova E, Ivanova-Smolenskaia I, Kurg A, Limborska S, Metspalu A. Association study of sporadic Parkinson's disease genetic risk factors in patients from Russia by APEX technology. Neurosci Lett. 2006;405:212–216. doi: 10.1016/j.neulet.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 12.Alberti KG, Zimmet PZ. WHO Consultation. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO Consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 13.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA, Duncan AW. Diagnosing insulin resistance in the general population. Diabetes Care. 2001;24:460–464. doi: 10.2337/diacare.24.3.460. [DOI] [PubMed] [Google Scholar]
- 17.Ranade K, Chang MS, Ting CT, Pei D, Hsiao CF, Olivier M, Pesich R, Hebert J, Chen YD, Dzau VJ. et al. High-throughput genotyping with single nucleotide polymorphisms. Genome Res. 2001;11:1262–1268. doi: 10.1101/gr.157801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider S, Roessli D, Excoffier L. Arlequin ver. 2.000: a software for population genetic data analysis. Genetics and Biometry Laboratory. University of Geneva; Switzerland: [Google Scholar]
- 19.STRAP - Interactive structure-based sequences alignment program. STRAP - Interactive structure-based sequences alignment program. http://3d-alignment.eu/
- 20.Sparso T, Andersen G, Albrechtsen A, Jorgensen T, Borch-Johnsen K, Sandbaek A, Lauritzen T, Wasson J, Permutt MA, Glazer B. et al. Impact of polymorphisms in WFS1 on prediabetic phenotypes in a population-based sample of middle-aged people with normal and abnormal glucose regulation. Diabetologia. 2008;51:1646–1652. doi: 10.1007/s00125-008-1064-2. [DOI] [PubMed] [Google Scholar]
- 21.Schäfer SA, Müssig K, Staiger H, Machicao F, Stefan N, Gallwitz B, Häring HU, Fritsche A. A common genetic variant in WFS1 determines impaired glucagon-like peptide-1-induced insulin secretion. Diabetologia. 2009;52:1075–1082. doi: 10.1007/s00125-009-1344-5. [DOI] [PubMed] [Google Scholar]
- 22.Heni M, Ketterer C, Thamer C, Herzberg-Schäfer SA, Guthoff M, Stefan N, Machicao F, Staiger H, Fritsche A, Häring HU. Glycemia determines the effect of type 2 diabetes risk genes on insulin secretion. Diabetes. 2010;59:3247–3252. doi: 10.2337/db10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mita M, Miyake K, Zenibayashi M, Hirota Y, Teranishi T, Kouyama K, Sakaguchi K, Kasuga M. Association study of the effect of WFS1 polymorphisms on risk of type 2 diabetes in Japanese population. Kobe J Med Sci. 2008;54:E192–E199. [PubMed] [Google Scholar]
- 24.Alimadadi A, Ebrahim-Habibi A, Abbasi F, Amoli MM, Sayahpour FA, Larijani B. Novel mutations of wolframin: a report with a look at the protein structure. Clin Genet. 2010;79:96–99. doi: 10.1111/j.1399-0004.2010.01511.x. [DOI] [PubMed] [Google Scholar]
- 25.Zatyka M, Ricketts C, da Silva Xavier G, Minton J, Fenton S, Hofmann-Thiel S, Rutter GA, Barrett TG. Sodium-potassium ATPase 1 subunit is a molecular partner of Wolframin, an endoplasmic reticulum protein involved in ER stress. Hum Mol Genet. 2008;17:190–200. doi: 10.1093/hmg/ddm296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishihara H, Takeda S, Tamura A, Takahashi R, Yamaguchi S, Takei D, Yamada T, Inoue H, Soga H, Katagiri H. et al. Disruption of the WFS1 gene in mice causes progressive beta-cell loss and impaired stimulus-secretion coupling in insulin secretion. Hum Mol Genet. 2004;13:1159–1170. doi: 10.1093/hmg/ddh125. [DOI] [PubMed] [Google Scholar]
- 27.Riggs AC, Bernal-Mizrachi E, Ohsugi M, Wasson J, Fatrai S, Welling C, Murray J, Schmidt RE, Herrera PL, Permutt MA. Mice conditionally lacking the Wolfram gene in pancreatic islet beta cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia. 2005;48:2313–2321. doi: 10.1007/s00125-005-1947-4. [DOI] [PubMed] [Google Scholar]
- 28.Yamada T, Ishihara H, Tamura A, Takahashi R, Yamaguchi S, Takei D, Tokita A, Satake C, Tashiro F, Katagiri H. et al. WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic beta-cells. Hum Mol Genet. 2006;15:1600–1609. doi: 10.1093/hmg/ddl081. [DOI] [PubMed] [Google Scholar]
- 29.Fonseca SG, Fukuma M, Lipson KL, Nguyen LX, Allen JR, Oka Y, Urano F. WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J Biol Chem. 2005;280:39609–39615. doi: 10.1074/jbc.M507426200. [DOI] [PubMed] [Google Scholar]
- 30.Takei D, Ishihara H, Yamaguchi S, Yamada T, Tamura A, Katagiri H, Maruyama Y, Oka Y. WFS1 protein modulates the free Ca(2+) concentration in the endoplasmic reticulum. FEBS Lett. 2006;580:5635–5640. doi: 10.1016/j.febslet.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Fonseca SG, Ishigaki S, Oslowski CM, Lu S, Lipson KL, Ghosh R, Hayashi E, Ishihara H, Oka Y, Permutt MA. et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J Clin Invest. 2010;120:744–755. doi: 10.1172/JCI39678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatanaka M, Tanabe K, Yanai A, Ohta Y, Kondo M, Akiyama M, Shinoda K, Oka Y, Tanizawa Y. Wolfram syndrome 1 gene (WFS1) product localizes to secretory granules and determines granule acidification in pancreatic beta-cells. Hum Mol Genet. 2011;20:1274–1284. doi: 10.1093/hmg/ddq568. [DOI] [PubMed] [Google Scholar]
- 33.Parikh H, Lyssenko V, Groop LC. Prioritizing genes for follow-up from genome wide association studies using information on gene expression in tissues relevant for type 2 diabetes mellitus. BMC Med Genomics. 2009;2:72. doi: 10.1186/1755-8794-2-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo X, Shen S, Song S, He S, Cui Y, Xing G, Wang J, Yin Y, Fan L, He F, Zhang L. The E3 ligase Smurf1 regulates Wolfram syndrome protein stability at the endoplasmic reticulum. J Biol Chem. 2011 doi: 10.1074/jbc.M111.225615. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


