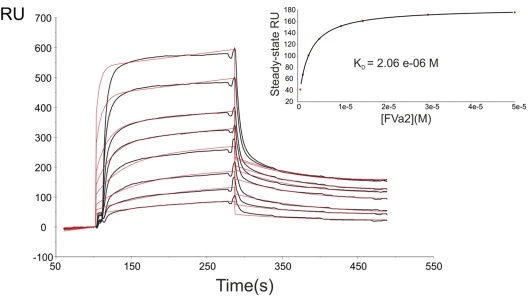
Figure 2.
The whole A2-B domain linker binds to thrombin with moderate affinity. Representative sensorgrams of the interaction between human FVa2 and immobilized FPR-thrombin are shown (black lines). Recombinant, highly purified FVa2 in HBS-EP buffer (10mM HEPES, pH 7.4, 150mM NaCl, 3mM EDTA, 0.005% [vol/vol] surfactant P20) was flowed over the surface of the FPR–α-thrombin–coated CM5 chip at 30 μL/min. Peptide concentrations from top to bottom were 50, 30, 15, 10, 5, 2.5, 1.2, and 0.6μM. Red lines overlaid with the sensorgrams represent the simultaneous fit by a 1:1 Langmuir binding model using nonlinear least-squares analysis with BIAevaluation 4.1 software. The results of steady-state analysis of the data are given in the inset. Data shown are representative of 4 independent experiments. RU indicates resonance units.