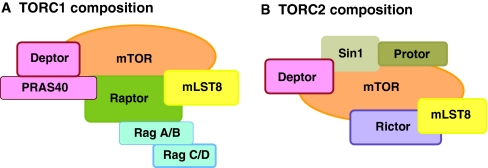
Fig. 2.
Composition of TORC1 and TORC2. (A) Target of rapamycin complex 1 (TORC1) (shown as a monomer) consists of mammalian TOR (mTOR), regulatory associated protein of mTOR (raptor), proline-rich AKT substrate 40 KDa (PRAS40), mammalian lethal with Sec-13 protein 8 (mLST8) and DEP domain TOR-binding protein (Deptor). Raptor interacts with some substrates and promotes dimerization of TORC1 complexes by direct interaction with TOR subunits from each monomer. PRAS40 binding to TORC1 is inhibitory and may be mediated by direct interaction with either mTOR or raptor. mLST8, via its multiple WD40 repeats, binds to the kinase domain of mTOR. Deptor binds the FAT (FRAP, ATM and TTRAP) domain of TOR and is capable of inhibiting both TORC1 and TORC2. Rag A/B and Rag C/D bind TORC1 through direct interaction with raptor. (B) TORC2 complex members include mTOR, rapamycin-insensitive companion of mTOR (Rictor), stress-activated protein kinase-interacting protein 1 (Sin1), mLST8, Deptor and protein-binding Rictor (protor). Rictor contains conserved domains that are hypothesized to be important for TORC2 complex formation and substrate recruitment. Sin1 has been described to promote Rictor-mTOR binding and regulate substrate specificity. Protor binds Rictor, although its function is currently unclear. mLST8 also binds mTOR and may be required for TORC2 function in vivo based on knockout data.