FIGURE 1.
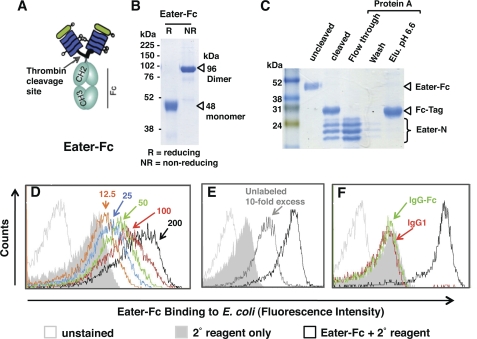
Baculovirus-expressed Eater-Fc fusion protein recognizes bacteria. A, schematic depiction of recombinant, secreted Eater-Fc protein. The putative ligand binding domain of Eater (amino acids 1–199 corresponding to two complete tandem EGF-like repeats) was fused to an Fc affinity tag. B and C, Coomassie Blue-stained SDS gels (12%). B, Eater-Fc fusion protein migrates as dimer under non-reducing conditions (NR). Expected molecular mass is indicated by open triangles. R, reducing conditions. C, thrombin-cleaved Eater-Fc and separation of cleavage products by protein A affinity chromatography. Cleaved Eater N-terminal fragment migrated in multiple bands, the lower of which corresponded to the predicted molecular mass (21 kDa). Elu. pH 6.6, elution buffer at pH 6.6. D–F. flow cytometry analysis of direct binding of biotinylated Eater-Fc fusion protein (200 μm; black open curve) to heat-inactivated E. coli. D, concentration-dependent binding (red, 100 μm; green, 50 μm; blue, 25 μm; orange, 12.5 μm). E, inhibition of binding by a 10-fold excess of non-biotinylated Eater-Fc (2 mm; dark gray curve). F, control. No significant binding activity was detected with biotinylated human IgG1 (200 μm; red line) or IgG-Fc (200 μm; green line). All experiments were repeated at least once with similar results.