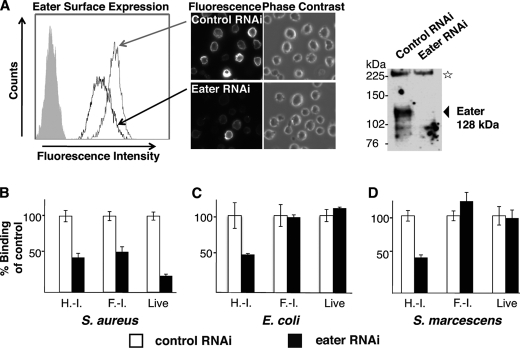
FIGURE 4.
Membrane-bound, native Eater receptor behaves similarly to Eater-Fc. A, specific cell surface staining of Eater on S2 cells. Polyclonal rabbit anti-Eater-Fc antibodies reacted with S2 cells by cell surface staining of live S2 cells (left panel, histogram of flow cytometry analysis; filled gray curve, secondary reagent only) or formaldehyde-fixed (non-permeabilized) S2 cells (central panels, microscopy, 40× magnification). Cell surface staining strongly diminished after Eater RNAi knockdown. In a Western blot of S2 cell lysate, anti-Eater-Fc antibodies recognized a specific band consistent with the predicted molecular mass of Eater (128 kDa; arrow) that disappeared after Eater RNAi knockdown (upper right panel). The star marks a nonspecific band serving as loading control. B–D, binding of bacteria to S2 cells, normalized to dsRNA-treated controls. B, binding of S2 cells to heat- or formaldehyde-inactivated and live S. aureus is partially Eater-dependent because the signal decreased after RNAi knockdown of Eater. C and D, binding of S2 cells to heat-inactivated Proteobacteria (E. coli, S. marcescens) was partially Eater-dependent (signal decrease after Eater-specific RNAi), whereas binding to formaldehyde-inactivated or live Proteobacteria was not Eater-dependent (no change in signal after Eater-specific RNAi). H.-I., heat-inactivated; F.-I., formaldehyde-inactivated. All experiments were repeated at least once with similar results.