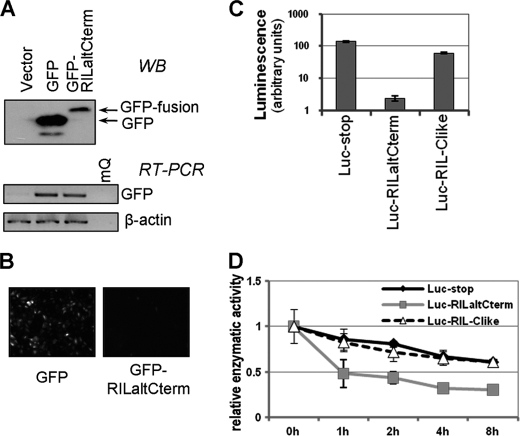
FIGURE 2.
Alternative C-terminal peptide of RIL destabilizes heterologous proteins. RIL alternative C-terminal peptide was fused in-frame with GFP and lentivirally expressed in A549 cells (A and B). A, abundance of modified GFP protein was significantly decreased (by Western blot (WB)), although mRNA levels remain comparable (RT-PCR). B, decreased fluorescence of cells expressing GFP fused to RIL alternative C-terminus compared with nonmodified GFP, as examined by fluorescence microscopy. C and D, luciferase was fused with RIL alternative C terminus in-frame (Luc-RILaltCterm) or with a frameshift (Luc-RIL-Clike) as a control and introduced into H1299 cells by transfection. C, Luc-RILaltCterm activity was severely impaired compared with controls (Luc-stop and Luc-RIL-Clike). Transfection efficiency was normalized for by β-galactosidase. D, cells were treated with 1 μg/ml cycloheximide for 0, 1, 2, 4, or 8 h, and stability of modified luciferase was assessed based on its enzymatic activity. Luc-RILaltCterm has a lower half-life time relative to controls.