Abstract
Objectives
The purpose of this analysis was to assess preoperative risk factors prior to the first-stage Norwood surgery in infants with hypoplastic left heart syndrome and related single ventricle lesions, and to evaluate practice patterns in prenatal diagnosis as well as the role of prenatal diagnosis in outcome.
Methods
Data from all live births with morphologic single right ventricle and systemic outflow obstruction screened for the Pediatric Heart Network Single Ventricle Reconstruction Trial were used to investigate prenatal diagnosis and preoperative risk factors. Demographics, gestational age, prenatal diagnosis status, presence of major extracardiac congenital abnormalities and preoperative mortality rates were recorded.
Results
Of 906 infants, 677 (75%) had prenatal diagnosis, 15% were preterm (<37 weeks), and 16% were low birth weight (<2500 g). Rates of prenatal diagnosis varied by study site (59%-85%, p<0.0001). Major extracardiac congenital abnormalities were less prevalent in those born after prenatal diagnosis (6% vs. 10%, p=0.03). There were 26 (3%) deaths prior to Norwood palliation; preoperative mortality did not differ by prenatal diagnosis status (p=0.49). In multiple logistic regression models, preterm birth (p=0.02), major extracardiac congenital abnormalities (p<0.0001), and obstructed pulmonary venous return (p=0.02) were independently associated with preoperative mortality.
Conclusions
Prenatal diagnosis occurred in 75%. Preoperative death was independently associated with preterm birth, obstructed pulmonary venous return and major extracardiac congenital abnormalities. Adjusted for gestational age and the presence of obstructed pulmonary venous return, the estimated odds of preoperative mortality were 10 times greater for subjects with a major extracardiac congenital abnormality.
Introduction
Diagnosis of congenital heart disease using fetal echocardiography was first reported in 1984,(1) and clinical use has been growing steadily.(2;3) Hypoplastic left heart syndrome (HLHS) and other related morphologic single right ventricle lesions with systemic outflow obstruction are among the most commonly prenatally diagnosed congenital heart defects.(4) Neonates with HLHS are often critically ill upon de novo presentation, and must be resuscitated and stabilized with prostaglandin E infusion before initial palliative surgery can be considered. Therefore, the assumption has been that prenatal diagnosis should improve outcome, but most studies have not demonstrated an improvement in postoperative survival with prenatal diagnosis.(5;6) A single study found that prenatal diagnosis was associated with improved preoperative clinical status and improved survival after the first-stage reconstruction (the Norwood procedure).(7) Considerable effort has been expended to identify risk factors for postoperative mortality following the Norwood palliation.(8;9) However, some infants die before reaching the operating room, and risk factors for preoperative mortality and potential associations between prenatal diagnosis, patient characteristics, and preoperative mortality are poorly understood. The aims of this investigation were to describe:
Current prevalence and timing of diagnosis (prenatal versus postnatal) in a large contemporary, multicenter cohort of patients with hypoplastic left heart syndrome or related morphologic single right ventricle lesions with systemic outflow obstruction
Center-level variation in the rate of prenatal diagnosis
Patient characteristics associated with prenatal diagnosis
Determinants of preoperative mortality including patient characteristics and prenatal diagnosis
Methods
Design of the Single Ventricle Reconstruction Trial
Data for the analyses were obtained as part of a screening protocol used in a multicenter randomized trial of modified Blalock-Taussig shunt (MBTS) versus right ventricular-to-pulmonary artery (RV-to-PA) shunt introduced during a stage-I palliation (Norwood) procedure. All live births with a diagnosis of a single morphologic right ventricle with systemic outflow obstruction were eligible for inclusion in the trial. The trial’s primary outcome measure was the composite proportion of subjects experiencing death or cardiac transplantation 12 months after randomization. The design of this trial, conducted by the National Heart, Lung, and Blood Institute (NHLBI)-funded Pediatric Heart Network (PHN) has been previously described.(10) From May 2005 to July 2009, 15 centers enrolled 555 subjects. (ClinicalTrials.gov number: NCT00115934)
The protocol was approved by an independent Protocol Review Committee and Data and Safety Monitoring Board, by institutional review boards at each clinical center, and at the Data Coordinating Center. All centers followed the same protocol and study procedures.
Screening of potential subjects
During the enrollment period, all neonates with a morphologic single right ventricle and systemic outflow obstruction admitted to participating centers were assessed for inclusion in the trial. Data collected at screening included birth weight, race, gender, gestational age, fetal intervention (atrial septostomy or aortic valve dilation), detailed cardiac diagnosis, presence of major extracardiac congenital abnormalities or acquired extracardiac disorders, and presence of and age at prenatal diagnosis. For the purposes of this study obstructed pulmonary venous return was defined by the use of postnatal intervention, including balloon septostomy, open atrial septectomy, or urgent Norwood procedure. Preoperative mortality was also recorded.
For the purposes of analysis, extracardiac congenital abnormalities were divided into chromosomal and non-chromosomal abnormalities. Chromosomal abnormalities included: trisomies 13, 18 and 21, Turner’s Syndrome, Ellis van Creveld Syndrome, Goldenhar Syndrome, Scimitar Syndrome, Jacobsen Syndrome, and other unidentified chromosomal abnormalities or genetic syndromes. Nonchromosomal abnormalities included acquired extracardiac disorders (e.g., meconium aspiration with need for high-frequency ventilation, persistent renal failure requiring dialysis) that the site investigator considered could independently affect likelihood of subject meeting primary endpoint of death or transplant at one year.(10)
Analytic sample and statistical analyses
Statistical analyses were performed using SAS software version 9.2 (SAS Institute, Cary, NC) and the R System, version 2.8.1 (R Foundation for Statistical Computing, Vienna, Austria). Exploratory analyses incorporating graphical and tabular displays were employed to assess bivariate associations. Sample means, medians and proportions accompanied by 95% confidence intervals were used to provide descriptive summaries. Chi-square tests of equality of proportions and Fisher exact test and Student’s t-test of equality of means were used to formally test hypotheses of no differences in various factors vs. prenatal diagnosis status. Simple and multiple logistic regressions were used to assess the relative strength of association between multiple risk factors and preoperative death. Backwards covariate selection with a significance criterion of 0.05 was employed to construct the multivariate logistic regression model. ANOVA and chi-square statistics were used to assess the degree of cross-center variation in the proportion of subjects with prenatal diagnosis and, among those subjects with prenatal diagnosis, fetal gestational age at that time. An adjusted screening population size was used for two centers that had abridged screening periods of participation to obtain center volume estimates based on total trial duration.
Results
Characteristics associated with prenatal diagnosis
From May 2005 to July 2009, 15 centers screened 921 neonates for the SVR trial; 15 were determined to not meet the study entry criteria of morphologic single right ventricle with systemic outflow obstruction and were excluded, leaving a total of 906 subjects for this analysis. Prenatal diagnosis was made in 677 (75%) of 906 subjects. Unadjusted associations between subject risk factors and prenatal diagnosis are presented in Table 1. The gender and racial/ethnic distributions of subjects with and without prenatal diagnosis were similar. Although the proportion of preterm infants did not differ by prenatal diagnosis status, subjects without a prenatal diagnosis were on average born at a higher gestational age than those with a prenatal diagnosis (mean 0.4 weeks; p=0.01). (Figure 1). Thirty-seven subjects did not have specific gestational age reported, but were classified as full term. These subjects were assigned the median gestational age among all subjects who were not preterm and whose gestational age was reported (38 wks). Subjects without prenatal diagnosis exhibited a greater prevalence of non-chromosomal extracardiac congenital abnormalities in comparison to subjects with prenatal diagnosis (9% vs. 4%, p = 0.01). Fetal intervention on the atrial septum or aortic valve was reported in 26 (3%) of the screened subjects. There were no differences in demographic factors, birth characteristics, presence of major extracardiac abnormalities or incidence of preoperative death between those with fetal intervention and the remainder of the cohort.
Table 1.
Demographic factors and other descriptors by Prenatal Status.
| Prenatal Diagnosis N(%)a or Mean ± SD | p-valb | ||
|---|---|---|---|
| Yes (N=677) | No (N=228) | ||
| Female | 265 (39%) | 85 (37%) | 0.64 |
| Race | |||
| White | 528 (78%) | 169 (74%) | 0.59 |
| Black / African American | 99 (15%) | 37 (16%) | |
| Asian | 16 (3%) | 6 (3%) | |
| Other | 34 (5%) | 16 (7%) | |
| Ethnicity | 0.28 | ||
| Hispanic | 117 (17%) | 49 (21%) | |
| non-Hispanic | 537 (80%) | 174 (76%) | |
| Unknown | 23 (3%) | 5 (2%) | |
| Major extracardiac congenital abnormalitya (N=904) | 38 (6%) | 22 (10%) | 0.03 |
| Chromosomalb | 10 (1%) | 2 (1%) | 0.74d |
| All otherb | 28 (4%) | 20 (9%) | 0.01 |
| Gestational Agec, wk | 37.8 ± 1.8 | 38.2 ± 2.0 | 0.01 |
| Preterm | 100 (15%) | 31 (14%) | 0.66 |
| Low Birth Weight (<2500 grams) | 115 (17%) | 31 (14%) | 0.23 |
| Birthweight, grams | 3038 ± 578 | 3081 ± 579 | 0.34 |
One subject with ‘unknown’ diagnosis status has been excluded.
Chi-square test or Student’s t-test (no equal variance assumption).
’Full term’ patients with unknown gestational age assigned gestational age 38 wk.
Fisher’s exact test.
Figure 1.
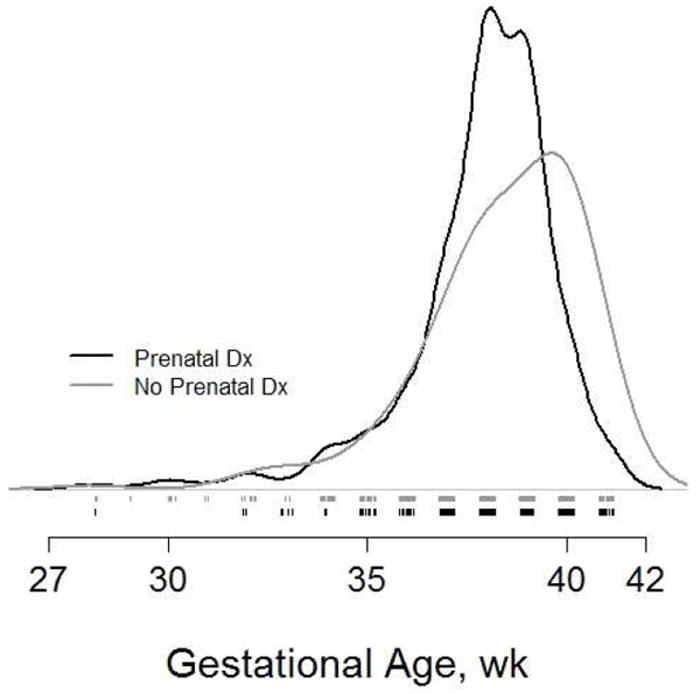
Kernel density estimates of gestational age according to prenatal diagnosis status.
Center-level variation
There was significant center-level variation in the proportion of subjects with prenatal diagnosis, ranging from 59% to 85% (p<0.0001, Figure 2). The positive association between the size of the center’s available screening population and the proportion of subjects with prenatal diagnosis is apparent. There was also significant center-level variation in mean fetal gestational age at diagnosis (range 20.6-29.5 weeks, p=0.01).
Figure 2.
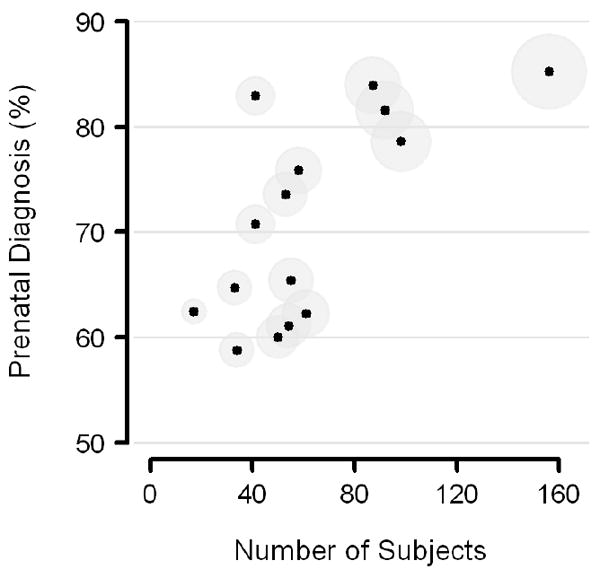
Center-specific proportions of subjects with prenatal diagnosis, versus number of subjects screened (N = 906). The 15 trial centers are displayed as circular symbols sized in relative proportion to the square root of their respective sample sizes.
Risk factors for preoperative mortality
There were 26 (3%) preoperative deaths. Univariable and multivariable associations between subject risk factors and preoperative death are presented in Table 2. Subjects who died prior to the Norwood procedure had significantly lower gestational age and birth weight and a much higher rate of extracardiac congenital anomalies compared with subjects who survived to the Norwood. In addition, obstructed pulmonary venous return was more common among non-survivors (23% vs. 5%, p=0.002).
Table 2.
Associations between risk factors and preoperative death.
| Mean ± SD or Percent | Unadjusted Associations | Multivariate Modelb | |||
|---|---|---|---|---|---|
| Yes (N=26) | No (N=880) | p-valuea | Odds Ratio (95% CI) | p-value | |
| Prenatal Diagnosis | 18 (69%) | 659 (75%) | 0.51 | ||
| Major extracardiac congenital abnormality | 12 (46%) | 48 (6%) | <0.0001 | 10.0 (4.0, 24.7) | <0.0001 |
| Chromosomal | 1 (4%) | 11 (1%) | 0.29 | ||
| All other | 11 (44%) | 37 (4%) | <0.0001 | ||
| Gestational Age, Weeks | 36 ± 3 | 38 ± 2 | 0.003 | 1.3 (1.1, 1.5) | 0.02 |
| Low Birth Weight (<2500 grams) | 11 (42%) | 136 (16%) | < 0.001 | ||
| Aortic Atresia | 16 (62%) | 530 (60%) | 0.90 | ||
| Obstructed Pulmonary Venous Return | 6 (23%) | 44 (5%) | 0.002 | 4.0 (1.3, 12.3) | 0.02 |
Chi-square test of equal proportions.
Multiple logistic regression (N=905). Only risk factors displaying significant univariate association with mortality are included
In multivariate logistic regression modeling, only major extracardiac congenital abnormalities, gestational age, and the presence or absence of obstructed pulmonary venous return remained significantly associated with preoperative mortality. (Table 2). The presence of a non-cardiac major congenital abnormality was most strongly associated with preoperative mortality (p < 0.0001). The estimated odds of preoperative mortality were 10 times greater for subjects with an extracardiac major congenital abnormality (95% CI: 4.1, 24.8) after controlling for gestational age and obstructed pulmonary venous return. Prenatal diagnosis was not a significant correlate of outcome when added to this model.
Discussion
The Single Ventricle Reconstruction Trial included systematic prospective screening data on the largest contemporary cohort of neonates with hypoplastic left heart syndrome and related single right ventricle lesions with systemic outflow obstruction to date. This has permitted an unprecedented assessment of the timing and occurrence of prenatal diagnosis, and risk factors for pre-operative death.
Characteristics associated with prenatal diagnosis
In contrast to data reported less than a decade ago with rates of prenatal diagnosis of less than 50%,(6;11) the majority (75%) of neonates screened for this trial had a prenatal diagnosis. These infants were born at a significantly lower gestational age than subjects diagnosed postnatally. However, while no difference was shown between diagnosis groups in the percentage born preterm (37 weeks), a considerably higher proportion of subjects in the postnatally diagnosed group were born at 39 weeks or later. The manner of delivery of subjects (natural labor, planned induction or scheduled caesarean section) was not collected, however we speculate that the overall difference in gestational age likely reflects a higher rate of planned induction of labor prior to 40 weeks’ gestation among the prenatal diagnosis group.
There is increasing appreciation of the association of extracardiac and chromosomal abnormalities and congenital heart disease.(12;13) In our study, major extracardiac congenital abnormalities occurred less frequently in the prenatal diagnosis group than in the postnatal diagnosis group, 6 vs. 10%, p=0.03, but interestingly this difference was driven by non-chromosomal anomalies as the proportion of chromosomal anomalies was similar in both groups. Zyblewski et al reported that women were 14 times more likely to terminate a pregnancy or seek comfort care for a fetus with congenital heart disease if a chromosomal abnormality was present. (13) By extension, the presence of extracardiac structural anomalies diagnosed during prenatal ultrasound may also influence parental treatment decisions. The lack of association between chromosomal anomalies and prenatal diagnosis in our study may be explained by a low rate of prenatal karyotyping in this cohort; however, this is speculation as this information was not collected. We have observed in clinical practice that despite strong physician recommendations in favor of prenatal karyotyping, many women decline amniocentesis out of concern of potential risk to the pregnancy. (14) It is important to note that differences in pregnancy termination rates in those with and without prenatal identification of more severe pathology may confound the association between prenatal diagnosis and preoperative mortality.
Center-level variation
Rates of prenatal diagnosis and timing (gestational age) of those diagnoses varied significantly by center. Figure 2 suggests a positive relationship between the number of subjects per site and the proportion of prenatal diagnoses. It seems plausible that prenatally diagnosed patients whose parents have decided in favor of neonatal surgical palliation may be referred preferentially to large tertiary care centers with established fetal cardiology practices and well known pediatric cardiac surgical programs. Center-level variation in gestational age at diagnosis could also be influenced by center variations in the number of patients choosing pregnancy termination or post-natal compassionate care only.
Risk factors for preoperative mortality
Reports concerning incidence and characteristics of preoperative mortality among infants with HLHS and related lesions are lacking. Mahle et al, found 4/216 (1.8%) of such subjects had preoperative death in a single center series.(6) In this multicenter cohort we found that 3% died before surgery. Infants with a major extracardiac congenital anomaly had a 10-fold higher risk of preoperative death, largely due to the presence of non-chromosomal anomalies. This finding is consistent with previous reports.(15) Previous reports suggest a positive a relationship between prenatal diagnosis and improved preoperative management in HLHS patients.(7;16;17) We, however, found no association between prenatal diagnosis and preoperative mortality. It is possible that improved education in neonatal units about the risks of ductal-dependent congenital heart disease in neonates has resulted in earlier recognition of those not prenatally diagnosed. However, the inverse relationship between prenatal diagnosis and extracardiac congenital abnormalities found in the current study may confound the analysis of any relationship between prenatal diagnosis and preoperative mortality.
Low birth weight is frequently mentioned in clinical discussions as a risk factor for surgical outcome, a view borne out in an analysis of data on several types of congenital heart operations from the Society of Thoracic Surgeons Congenital Heart Database. (18) We were surprised, therefore, by the finding that gestational age, but not birth weight, was a significant predictor of preoperative mortality in multivariable analyses. We know from a recent analysis of growth parameters in the population screened for the PHN’s randomized controlled trial of enalapril vs. placebo in infants with single ventricle, that there is a significantly higher rate of growth retardation in infants with single ventricle physiology compared to that of the general population. (19) This suggests that, at a given gestational age, infants with single ventricle physiology are smaller than infants with normal hearts. The physiological maturation implicit in increased gestational age may be more significant for fetal and neonatal well-being than size per se. The association between lower gestational age and increased mortality after infant heart surgery supports the importance of gestational age.(20) This finding deserves further evaluation and consideration in risk-stratification scores.
Obstructed pulmonary venous return is a well-known risk factor for postoperative mortality following the Norwood.(9) We found this also to be a risk factor for preoperative mortality. Moreover, our findings are consistent with previous studies that showed no benefit of prenatal diagnosis of atrial level restriction on outcome.(21) Invasive decompression of the left atrium in fetuses with HLHS and a restrictive atrial septum has been reported;(22) whether this strategy will improve outcomes remains to be seen.
The limitations of this study are primarily related to potential selection bias. Only live subjects born at or referred to one of the 15 participating surgical centers were included. These centers may not be representative of all centers performing complex neonatal surgery in North America. In addition, within the 15 trial centers, selection bias may have occurred as a result of variation in pregnancy termination following prenatal diagnosis. More detailed information on the causes of preoperative death was not available; therefore some deaths may have been related to decisions not to pursue intervention. Finally, this study is restricted to events occurring prior to Norwood surgery; assessment of the impact of prenatal diagnosis on postoperative survival and other outcomes awaits trial completion.
Summary
Using prospective screening data on the largest contemporary cohort of neonates with HLHS and related single right ventricle lesions to date, we found that prenatal diagnosis by fetal echocardiography occurred in 75%. Non-chromosomal major congenital anomalies were less common among those with a prenatal diagnosis. Preoperative death occurred in 3% of subjects and was independently associated with preterm birth, obstructed pulmonary venous return and major extracardiac congenital abnormalities. Adjusted for gestational age and the presence or absence of obstructed pulmonary venous return, the estimated odds of preoperative mortality were 10 times greater for subjects with a major extracardiac congenital abnormality.
Acknowledgments
Supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288, HL085057)
APPENDIX
National Heart, Lung, and Blood Institute
Gail Pearson, Victoria Pemberton, Rae-Ellen Kavey, Mario Stylianou, Marsha Mathis.
Network Chair
University of Texas Southwestern Medical Center, Lynn Mahony
Data Coordinating Center
New England Research Institutes, Lynn Sleeper (PI), Sharon Tennstedt (PI), Steven Colan, Lisa Virzi, Patty Connell, Victoria Muratov, Lisa Wruck, Minmin Lu, Dianne Gallagher, Anne Devine, Thomas Travison, David F. Teitel
Core Clinical Site Investigators
Children’s Hospital Boston, Jane W. Newburger (PI), Peter Laussen, Pedro del Nido, Roger Breitbart, Jami Levine, Ellen McGrath, Carolyn Dunbar-Masterson; Children’s Hospital of New York , Wyman Lai (PI), Beth Printz (currently at Rady Children’s Hospital), Daphne Hsu (currently at Montefiore Medical Center), William Hellenbrand, Ismee Williams, Ashwin Prakash, Ralph Mosca, Darlene Servedio, Rozelle Corda, Rosalind Korsin, Mary Nash; Children’s Hospital of Philadelphia, Victoria L. Vetter (PI), Sarah Tabbutt (currently at the University of San Francisco), J. William Gaynor (Study Co-Chair), Chitra Ravishankar, Thomas Spray, Meryl Cohen, Marisa Nolan, Stephanie Piacentino, Sandra DiLullo, Nicole Mirarchi; Cincinnati Children’s Medical Center, D. Woodrow Benson (PI), Catherine Dent Krawczeski, Lois Bogenschutz, Teresa Barnard, Michelle Hamstra, Rachel Griffiths, Kathie Hogan, Steven Schwartz, David Nelson; North Carolina Consortium: Duke University, East Carolina University, Wake Forest University, Page A. W. Anderson (PI) – deceased, Jennifer Li (PI), Wesley Covitz, Kari Crawford, Michael Hines, James Jaggers, Theodore Koutlas, Charlie Sang, Jr., Lori Jo Sutton, Mingfen Xu; Medical University of South Carolina, J. Philip Saul (PI), Andrew Atz, Girish Shirali, Scott Bradley, Eric Graham, Patricia Infinger; Primary Children’s Medical Center and the University of Utah, Salt Lake City, Utah, L. LuAnn Minich (PI), John Hawkins, Michael Puchalski, Richard Williams, Linda Lambert, Jun Porter, Marian Shearrow; Hospital for Sick Children, Toronto, Brian McCrindle (PI), Joel Kirsh, Chris Caldarone, Elizabeth Radojewski, Svetlana Khaikin, Susan McIntyre,Nancy Slater; University of Michigan, Caren S. Goldberg (PI), Richard G. Ohye (Study Chair), Cheryl Nowak; Children’s Hospital of Wisconsin, Nancy Ghanayem (PI), James Tweddell, Kathy Mussatto, Michele Frommelt, Lisa Young-Borkowski
Auxiliary Sites
Children’s Hospital Los Angeles, Alan Lewis (PI), Vaughn Starnes, Nancy Pike; The Congenital Heart Institute of Florida (CHIF), Jeffrey P. Jacobs, MD (PI), James A. Quintessenza, Paul J. Chai, David S. Cooper, J. Blaine John, James C. Huhta, Tina Merola, Tracey Cox; Emory University , Kirk Kanter, William Mahle, Joel Bond, Leslie French, Jeryl Huckaby; Nemours Cardiac Center, Christian Pizarro, Carol Prospero; Julie Simons, Gina Baffa; University of Texas Southwestern Medical Center, Ilana Zeltzer (PI), TiaTortoriello, Deborah McElroy, Deborah Town.
Angiography core laboratory
Duke University, John Rhodes, J. Curt Fudge
Echocardiography core laboratories
Children’s Hospital of Wisconsin, Peter Frommelt; Children’s Hospital Boston, Gerald Marx.
Genetics Core Laboratory
Children’s Hospital of Philadelphia, Catherine Stolle.
Protocol Review Committee
Michael Artman (Chair); Erle Austin; Timothy Feltes, Julie Johnson, Thomas Klitzner, Jeffrey Krischer, G. Paul Matherne.
Data and Safety Monitoring Board
John Kugler (Chair); Rae-Ellen Kavey, Executive Secretary; David J. Driscoll, Mark Galantowicz, Sally A. Hunsberger, Thomas J. Knight, Holly Taylor, Catherine L. Webb
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Allan LD, Crawford DC, Anderson RH, Tynan MJ. Echocardiographic and anatomical correlations in fetal congenital heart disease. Br Heart J. 1984 Nov;52(5):542–8. doi: 10.1136/hrt.52.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang RK, Chen AY, Klitzner TS. Clinical management of infants with hypoplastic left heart syndrome in the United States, 1988-1997. Pediatrics. 2002 Aug;110(2 Pt 1):292–8. doi: 10.1542/peds.110.2.292. [DOI] [PubMed] [Google Scholar]
- 3.Rasiah SV, Ewer AK, Miller P, Wright JG, Barron DJ, Brawn WJ, et al. Antenatal perspective of hypoplastic left heart syndrome: 5 years on. Arch Dis Child Fetal Neonatal Ed. 2008 May;93(3):F192–F197. doi: 10.1136/adc.2006.112482. [DOI] [PubMed] [Google Scholar]
- 4.Allan LD, Sharland GK, Milburn A, Lockhart SM, Groves AM, Anderson RH, et al. Prospective diagnosis of 1,006 consecutive cases of congenital heart disease in the fetus. J Am Coll Cardiol. 1994 May;23(6):1452–8. doi: 10.1016/0735-1097(94)90391-3. [DOI] [PubMed] [Google Scholar]
- 5.Kumar RK, Newburger JW, Gauvreau K, Kamenir SA, Hornberger LK. Comparison of outcome when hypoplastic left heart syndrome and transposition of the great arteries are diagnosed prenatally versus when diagnosis of these two conditions is made only postnatally. Am J Cardiol. 1999 Jun 15;83(12):1649–53. doi: 10.1016/s0002-9149(99)00172-1. [DOI] [PubMed] [Google Scholar]
- 6.Mahle WT, Clancy RR, McGaurn SP, Goin JE, Clark BJ. Impact of prenatal diagnosis on survival and early neurologic morbidity in neonates with the hypoplastic left heart syndrome. Pediatrics. 2001 Jun;107(6):1277–82. doi: 10.1542/peds.107.6.1277. [DOI] [PubMed] [Google Scholar]
- 7.Tworetzky W, McElhinney DB, Reddy VM, Brook MM, Hanley FL, Silverman NH. Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation. 2001 Mar 6;103(9):1269–73. doi: 10.1161/01.cir.103.9.1269. [DOI] [PubMed] [Google Scholar]
- 8.Sano S, Huang SC, Kasahara S, Yoshizumi K, Kotani Y, Ishino K. Risk factors for mortality after the Norwood procedure using right ventricle to pulmonary artery shunt. Ann Thorac Surg. 2009 Jan;87(1):178–85. doi: 10.1016/j.athoracsur.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 9.Stasik CN, Gelehrter S, Goldberg CS, Bove EL, Devaney EJ, Ohye RG. Current outcomes and risk factors for the Norwood procedure. J Thorac Cardiovasc Surg. 2006 Feb;131(2):412–7. doi: 10.1016/j.jtcvs.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 10.Ohye RG, Gaynor JW, Ghanayem NS, Goldberg CS, Laussen PC, Frommelt PC, et al. Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2008 Oct;136(4):968–75. doi: 10.1016/j.jtcvs.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fountain-Dommer RR, Bradley SM, Atz AM, Stroud MR, Forbus GA, Shirali GS. Outcome following, and impact of, prenatal identification of the candidates for the Norwood procedure. Cardiol Young. 2004 Feb;14(1):32–8. doi: 10.1017/s1047951104001064. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez JH, Shirali GS, Atz AM, Taylor SN, Forbus GA, Zyblewski SC, et al. Universal screening for extracardiac abnormalities in neonates with congenital heart disease. Pediatr Cardiol. 2009 Apr;30(3):269–73. doi: 10.1007/s00246-008-9331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009 Mar;137(3):529–36. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zyblewski SC, Hill EG, Shirali G, Atz A, Forbus G, Gonzalez J, et al. Chormosonal anomaies influence parental treatment decisions in relation to prenatally diagnosed congenital heart disease. Pediatr Cardiol. 2009 Nov;30(8):1105–11. doi: 10.1007/s00246-009-9514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wald RM, Tham EB, McCrindle BW, Goff DA, McAuliffe FM, Golding F, et al. Outcome after prenatal diagnosis of tricuspid atresia: a multicenter experience. Am Heart J. 2007 May;153(5):772–8. doi: 10.1016/j.ahj.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Tibballs J, Cantwell-Bartl A. Outcomes of management decisions by parents for their infants with hypoplastic left heart syndrome born with and without a prenatal diagnosis. J Paediatr Child Health. 2008 Jun;44(6):321–4. doi: 10.1111/j.1440-1754.2007.01265.x. [DOI] [PubMed] [Google Scholar]
- 17.Verheijen PM, Lisowski LA, Stoutenbeek P, Hitchcock JF, Brenner JI, Copel JA, et al. Prenatal diagnosis of congenital heart disease affects preoperative acidosis in the newborn patient. J Thorac Cardiovasc Surg. 2001 Apr;121(4):798–803. doi: 10.1067/mtc.2001.112825. [DOI] [PubMed] [Google Scholar]
- 18.Curzon CL, Milford-Beland S, Li JS, O’Brien SM, Jacobs JP, Jacobs ML, et al. Cardiac surgery in infants with low birth weight is associated with increased mortality: analysis of the Society of Thoracic Surgeons Congenital Heart Database. J Thorac Cardiovasc Surg. 2008 Mar;135(3):546–51. doi: 10.1016/j.jtcvs.2007.09.068. [DOI] [PubMed] [Google Scholar]
- 19.Williams R, Ravishankar C, Zak V, Atz A, Border W, Levine J, et al. Screening data from the Pediatric Heart Network Infant Single Venricle Trial: Association of single ventricle physiology with preterm birth and low birth weight. Congenit Heart Dis. 2008;5:96–103. doi: 10.1111/j.1747-0803.2009.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costello JM, Polito A, Brown DW, McElrath TF, Graham DA, Thiagarajan RR, et al. Timing of delivery and outcomes in neonates with critical congenital heart disease. Circulation. 2009;120:S585. [Google Scholar]
- 21.Glatz JA, Tabbutt S, Gaynor JW, Rome JJ, Montenegro L, Spray TL, et al. Hypoplastic left heart syndrome with atrial level restriction in the era of prenatal diagnosis. Ann Thorac Surg. 2007 Nov;84(5):1633–8. doi: 10.1016/j.athoracsur.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 22.Vida VL, Bacha EA, Larrazabal A, Gauvreau K, Thiagaragan R, Fynn-Thompson F, et al. Hypoplastic left heart syndrome with intact or highly restrictive atrial septum: surgical experience from a single center. Ann Thorac Surg. 2007 Aug;84(2):581–5. doi: 10.1016/j.athoracsur.2007.04.017. [DOI] [PubMed] [Google Scholar]


