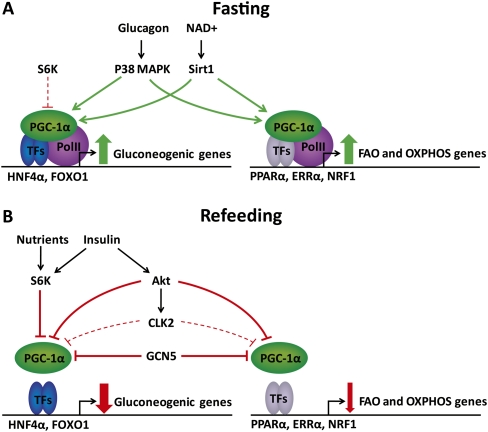
Figure 1.
Post-translational regulation of PGC-1α activity in liver during fasting and refeeding. (A) During fasting, glucagon and NAD+ activate PGC-1α through MAPK p38-mediated phosphorylation and Sirt1-mediated deacetylation. Low levels of S6K may be involved in feedback regulation of gluconeogenesis by specifically interfering with PGC-1α coactivation of HNF4α. (B) After refeeding, increased nutrient levels activate S6K1, which may lead to an acute, specific repression of gluconeogenic gene expression by phosphorylation of PGC-1α. Furthermore, the nutrient-induced rise in insulin activates the Akt kinase, which phosphorylates PGC-1α, leading to repression of gene programs involved in gluconeogenesis, fatty acid oxidation (FAO), and oxidative phosphorylation (OXPHOS). In addition, Akt phosphorylates the CLK2 kinase, which promotes sustained repression of predominantly gluconeogenic genes. Finally, refeeding leads to increased expression of steroid receptor coactivator 3 (SRC3) and general control of amino acid synthesis 5 (GCN5), which acetylates and thereby inactivates PGC1-α.