Figure 2.
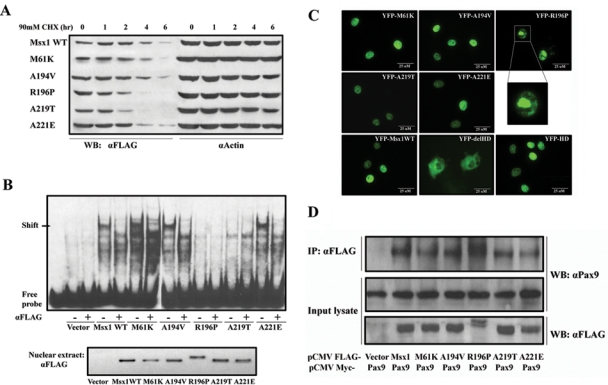
Characterizations of Msx1 protein. (A) Msx1 protein half-life: Cos7 cells were transfected with Flag-Msx1 expression vectors and treated with 90 mM cycloheximide (CHX) for 1 to 6 hrs. Western blot of cell lysates with anti-FLAG was used to assess the protein degradation speed; actin served as a control. (B) DNA-binding abilities: Electrophoretic mobility shift assay (EMSA) was used to test DNA-binding activity of nuclear extracts from Flag-Msx1-transfected COS7 cells with a double-stranded oligonucleotide probe containing a consensus binding-site for Msx1. Specificity of the complexes was confirmed by super-shift induction with anti-FLAG antibody. To verify that equal amounts of Msx1 protein were used for the EMSA, we performed a Western blot of nuclear extracts (lower panel). (Note that R196P migrates more slowly than the other proteins.) (C) Nuclear localization: YFP-Msx1 fusion proteins were expressed in COS7 cells. None of the missense mutations affected nuclear localization, in contrast to the YFP-Msx1 construct without homeodomain, which is found in the cytoplasm (YFP-delHD). The homeodomain by itself suffices for translocation to the nucleus (YFP-HD). YFP-R196P showed densely clumped distribution within the nuclei. These protein aggregates could be the consequence of protein interactions or of a secondary modification which also causes the R196P mutant to migrate more slowly than the other mutants and wild type Msx1. (D) Protein interaction: Co-immunoprecipitation with COS7 cells co-transfected with Myc-tagged Pax9 proteins and FLAG-tagged Msx1. Upper panel: Western blot analyses of immunoprecipitates of whole-cell lysates pulled down with anti-FLAG antibody and probed with an anti-Pax9 antibody. Middle panel: Whole-cell lysates probed with anti-Pax9 antibody or (lower panel) anti-Flag antibody. (Note that the Msx1-R196P migrates more slowly in both immunoprecipitations and input cell lysate.)
