Abstract
Bisphosphonate-related osteonecrosis of the jaw (BRONJ) commonly occurs in individuals receiving bisphosphonates (BPs) with clinical manifestations of the exposed necrotic bone. Although defective wound healing of soft tissue is frequently, if not always, observed in BRONJ, the effects of BPs on oral soft tissue or cells remain unknown. To investigate the effects of BPs on cells of oral mucosal tissue, we studied the effect of pamidronate (PAM), one of the BPs most commonly administered to cancer patients, on the phenotypes of normal human oral keratinocytes (NHOK) and fibroblasts (NHOF). When exposed to PAM at 10 µM, NHOK, not NHOF, underwent senescence: NHOK overexpressed senescence-associated β-galactosidase (SA-β-Gal), p16INK4A, IL-6, and IL-8. When exposed to a higher level (50 µM) of PAM, NHOK maintained senescent phenotypes, but NHOF underwent apoptosis. PAM-induced senescence in NHOK is mediated, in part, via geranylgeranylation of the mevalonate pathway. Our in vitro 3D oral mucosal tissue construction studies further demonstrated that PAM induced senescence and impaired re-epithelialization of oral mucosa. Analysis of these data indicates that premature senescence of oral mucosal cells and subsequent defective soft-tissue wound healing might be partly responsible for the development of BRONJ in individuals receiving PAM or other BPs.
Keywords: bisphosphonates, apoptosis, senescence, oral fibroblasts, oral keratinocytes
Introduction
Bisphosphonates (BPs) are potent anti-resorptive agents that are widely used to treat osteoporosis and metastatic bone diseases. With the increasing use of BPs due to their effectiveness in multiple clinical settings, osteonecrosis of the jaw (ONJ) has surfaced as a significant health problem. Approximately 80% of bisphosphonate-related ONJ (BRONJ) is due to invasive dental procedures including tooth extractions (Van den Wyngaert et al., 2006), and pressure due to denture prostheses has also been suggested as a precipitating factor (Levin et al., 2007), implicating dental trauma in BRONJ.
Clinically, BRONJ is characterized by chronically exposed necrotic bone, and defective wound healing of soft tissue is frequently, if not always, observed in BRONJ. A growing body of evidence supports a notion that BRONJ may be associated with soft-tissue toxicity (Reid et al., 2007; Landesberg et al., 2008; Scheper et al., 2009). One of the major side-effects of orally administered BP is cytotoxicity of the gastrointestinal (GI) tract, which is lined with stratified epithelium. Esophageal inflammation and ulceration are frequently reported in BP users (de Groen et al., 1996; Watts and Becker, 1999; Marshall et al., 2006), and oral ulceration is also observed in individuals taking oral BP tablets (Rubegni and Fimiani, 2006; Adornato et al., 2007). These reports suggest that BP may have direct effects on soft tissues; however, the role of oral mucosal soft tissue in the pathophysiology of BRONJ is poorly understood.
To embark on elucidating the role of oral mucosal soft tissue in BRONJ, we investigated the phenotypic effects of BPs on normal oral mucosal cells of human origin. We hypothesize that BPs elicit differential cellular responses in normal human oral keratinocytes (NHOK) and fibroblasts (NHOF). In this study, we report that pamidronate (PAM) asserts its cytotoxic effects on NHOF mainly through apoptosis. We also report, for the first time, that PAM induces senescence in NHOK in the mevalonate pathway-dependent manner via geranylgeranylation. Our 3-dimensional (3D) oral mucosal tissue constructs also demonstrated that PAM induces senescence and impairs proliferation and migration, two processes that are important for the re-epithelialization of the oral mucosal cells. These findings suggest that the development of BRONJ in persons receiving PAM or other BPs may be associated with BP-induced premature senescence of oral mucosal cells and subsequent defective soft-tissue wound healing.
Materials & Methods
Cells and Cell Culture
Primary NHOK and NHOF were prepared from oral mucosal tissues according to methods described elsewhere (Kang et al., 1998). NHOK were cultured in EpiLife supplemented with HKGS (Cascade Biologics, Portland, OR, USA), and NHOF were cultured in DMEM/199 (4:1) (Invitrogen, Carlsbad, CA, USA) with 10% Super Calf Serum (Gemini Bio-Products, Calabasas, CA, USA). PAM was purchased from LKT Labora-tories, Inc. (St. Paul, MN, USA). Geranylgeraniol (GGOH), farnesol (FOH), and lovastatin (Lov) were purchased from Sigma (St. Louis, MO, USA).
Cell Cycle and Apoptosis Analysis
PAM-treated cells were harvested and re-suspended in the hypotonic DNA staining buffer (3.4 mM sodium citrate, 0.3% Triton X, 20 µg/mL ribonuclease A, and 100 µg/mL propidium iodide) for 30 min to 1 hr at 4°C, and processed for cell cycle analysis. For the apoptosis analysis, an FITC Annexin V Apoptosis Detection Kit (BD Bioscience, San Jose, CA, USA) was used according to the manufacturer’s protocol. The data were obtained by use of the flow cytometer in the UCLA Flow Cytometry Core Laboratory.
Western Blotting
Cells were lysed and subjected to Western blotting as described previously (Kim et al., 2010). The following antibodies were used: caspase-3 (H-277), p16 (C-20), GAPDH (FL335), and β-actin (I-19) from Santa Cruz Biotechnology (Santa Cruz, CA, USA); and phospho-p38 (9211) and p38 (#9212) from Cell Signaling (Danvers, MA, USA).
Senescence-associated β-galactosidase (SA-β-Gal) Staining
SA-β-Gal staining was performed as previously described (Kim et al., 2010). For quantification, 1000 cells that included both β-Gal-positives and -negatives were counted in a random manner by a third person, and the percentages of β-Gal-positive cells were obtained. We also used a MarkerGene Cellular Senescence Microtiterplate Assay Kit (MarkerGene Technologies, Inc., Eugene, OR, USA) according to the manufacturer’s protocol.
Organotypic Raft Culture
A 3D oral mucosal wound-healing model was established by the organotypic raft culture system as described previously (Chen et al., 2010). Briefly, a mixture of NHOF and collagen was plated in a transwell. After 3 days, NHOK were plated on top of the stromal-like collagen bed, and cells were grown for another 4 days until the transwell containing the NHOF/NHOK bed was “air-lifted”. Four days after the transwell was air-lifted, the oral mucosal tissue constructs were continuously fed with medium only (CTL), medium containing 10 µM PAM, or medium containing 10 µM Lov every 3 days. The oral mucosal tissue constructs were harvested 14 days after the air-lifting and were formalin-fixed, paraffin-embedded (FFPE), and stained with hematoxylin and eosin (H&E). For the wound-healing model, tissue constructs were air-lifted for 4 days, after which they were treated with PAM or Lov. Circular “wounds” were created by means of a biopsy punch at the seventh day after air-lifting, and tissue constructs were harvested 14 days after air-lifting. Slides were scanned at 100x magnification.
Immunohistochemical (IHC) Staining
IHC staining was performed as described previously (Shin et al., 2006). PCNA antibody (Ab-1, Calbiochem, San Diego, CA, USA) was used at 1:200. Stained tissues were developed with the 3,3′-diaminobenzidine (DAB) chromogen substrate (Vector Laboratories Inc., Burlingame, CA, USA). For quantification, 3 independent fields were randomly selected, and PCNA-positive cells were counted per mm2. The bar represents the standard deviation.
Results
Pamidronate Induces Cytotoxic Effects on Oral Mucosal Cells in a Cell-type-specific Manner
To examine direct effects of oral mucosal cells by bisphosphonates, we first performed MTT assay with various doses of PAM for 4 days and examined the cell viability. We found a gradual decrease in cell viability in NHOK, starting as low as 1 µM PAM, whereas an abrupt decrease was evident in NHOF at approximately 25 µM PAM (Fig. 1A). Using 10 µM PAM, we found a significant loss of proliferation in NHOK with rounded and flattened phenotypes (Figs. 1B,1C; Appendix Materials & Methods). No phenotypic alterations were observed in NHOF at 10 µM PAM. The cell cycle analysis revealed an increased S-phase content in response to both 10 and 50 µM PAM doses in NHOK (Figs. 1D,1E). In contrast, NHOF exhibited no changes at 10 µM PAM but a significant increase in sub-G1 content at 50 µM PAM, suggesting that these cells underwent apoptosis at higher doses. Analysis of these data, collectively, suggests that PAM induces cytotoxic effects on oral mucosal cells in a cell-type-specific manner.
Figure 1.
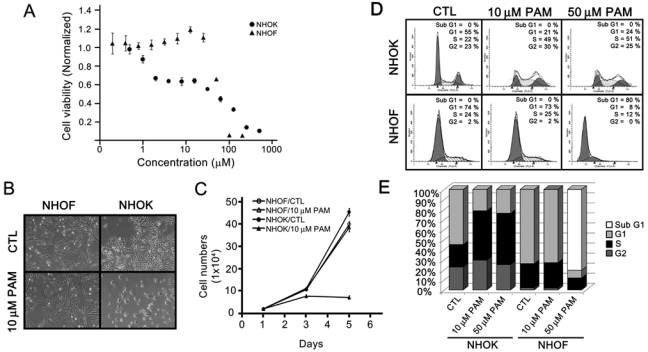
Pamidronate induces cytotoxic effects on oral mucosal cells in a cell-type-specific manner. (A) NHOK and NHOF were treated with various doses of pamidronate (PAM). Four days after treatment, cells were subjected to MTT assay. Experiments were performed in quadruplicate, and bars indicate standard errors. (B) NHOK and NHOF were treated with 10 µM PAM for 4 days, and cells were photographed at 100X. (C) NHOK and NHOF were plated onto the 6-well plates 24 hrs before cells were treated with 10 µM PAM. Every 2 days, cells were either harvested for counting or changed with fresh medium containing 10 µM PAM. (D) NHOK and NHOF were treated with 10 and 50 µM PAM for 4 days, and cells were harvested, stained with PI, and subjected to flow cytometry analysis. (E) The percentages of cell cycle contents in Fig. 1D were quantitated and are presented with the bar graph.
Pamidronate Induces Senescence, But Not Apoptosis, in NHOK
We further examined apoptotic responses using Annexin V flow analysis. In NHOK, apoptotic response was marginally increased in response to 10 µM and 50 µM of PAM (Figs. 2A, 2B). On the contrary, NHOF showed no, if not decreased, apoptotic response to 10 µM PAM treatment; however, a significant apoptotic response was noted in NHOF treated with 50 µM PAM, which was consistent with our cell cycle analysis (Figs. 1D,1E). Western blotting against cleaved caspase-3 showed similar results (Fig. 2C), suggesting that the cytotoxic effect of PAM in NHOF, but not in NHOK, is mediated through apoptosis. Because PAM did not induce apoptosis in NHOK, we examined whether PAM induces senescence, an alternative cellular mechanism by which cells become growth-arrested and acquire resistance to apoptosis (Qin et al., 2002; Chaturvedi et al., 2004). We performed SA-β-Gal staining and Western blotting against p16 and phosphorylated p38, all of which are markers for senescence. In PAM-treated NHOK, SA-β-gal activity increased up to 3.5-fold compared with that in the control, whereas no changes were observed in NHOF (Figs. 2D,2E). The expression of p16, phosphorylated p38, and the senescence-associated cytokines such as IL-6 and IL-8 (Freund et al., 2010) was induced by PAM in NHOK (Figs. 2F,2G,2H,2I; Appendix Materials & Methods). Analysis of these data indicates that PAM induces senescence, but not apoptosis, in NHOK.
Figure 2.
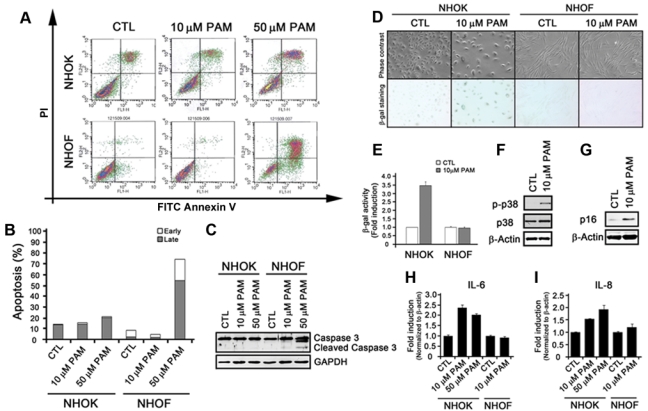
Pamidronate induces senescence, but not apoptosis, in NHOK. (A) NHOK and NHOF were treated with 10 and 50 µM PAM for 4 days, stained with FITC Annexin V and PI, and subjected to Flow Cytometry analysis. (B) The percentages of cells in early and late apoptosis in Fig. 2A were quantitated and are presented in the bar graph. (C) NHOK and NHOF were treated with 10 and 50 µM for 4 days, and cells were harvested for Western blot analysis against cleaved Caspase-3. GAPDH was also probed for a loading control. (D) NHOK and NHOF were treated with 10 µM PAM for 4 days and stained for SA-β-Gal activity. The photographs were taken at 100X. (E) β-Gal-positive NHOK and NHOF were quantitated by use of the MarkerGene Cellular Senescence Microtiterplate Assay Kit. (F) PAM-treated NHOK were subjected to Western blotting against phosphorylated p38 and total p38. β-actin was probed for the loading control. (G) Western blotting against p16 was also probed. (H, I) NHOK and NHOF treated with 10 or 50 µM PAM were harvested, mRNA was isolated, and cDNA was synthesized. qRT-PCR was run with primers for IL-6 and IL-8. Samples were run in triplicate and normalized to β-actin.
Reconstituting Geranylgeranylation, But Not Farnesylation, Partially Recovers Proliferation and Reduces Senescence in PAM-treated NHOK
At the molecular level, BPs physically bind to and inhibit the enzymatic activity of farnesyl pyrophosphate (FFPS), a key branch-point enzyme in the mevalonate pathway that leads to cholesterol synthesis and isoprenylation such as geranylgeranylation and farnesylation (Reszka and Rodan, 2004). To delineate whether senescence is induced via the mevalonate pathway, we reconstituted geranylgeranylation and farnesylation using GGOH and FOH, respectively, and examined proliferation and senescence. Sublethal amounts of FOH, GGOH, and FOH/GGOH (1:0.5 ratio) were determined by MTT assay (Appendix Fig. 1). Proliferation of PAM-treated NHOK was inhibited as expected. When NHOK were co-treated with PAM and FOH, similar results were observed; however, NHOK treated with PAM/GGOH or PAM/GGOH/FOH regained proliferative capacity by approximately half (Figs. 3A,3B). When these cells were subjected to SA-β-gal staining, GGOH, but not FOH, reduced β-gal positivity by approximately half (Fig. 3C). Analysis of these data suggests that the senescence mechanism in PAM-treated NHOK is mediated, in part, through geranylgeranylation of the mevalonate pathway.
Figure 3.
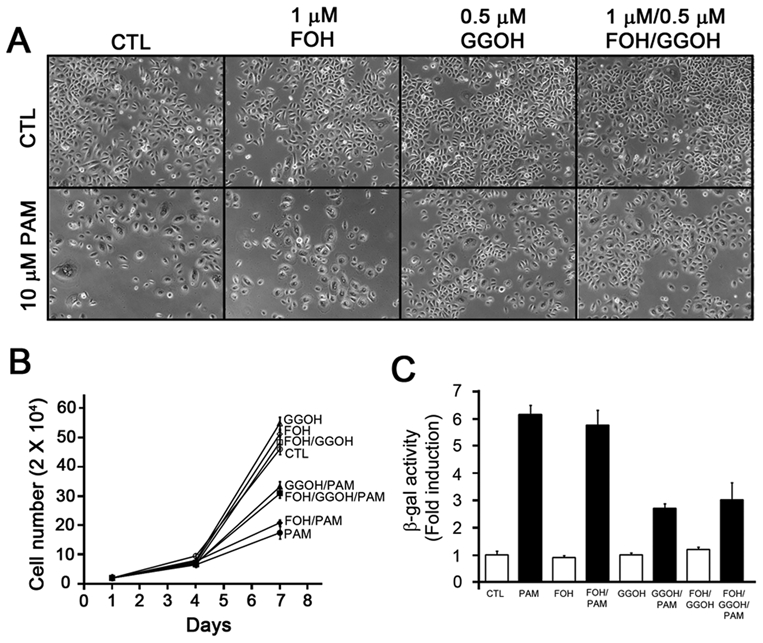
Geranylgerniol (GGOH), but not farnesol (FOH), partially reverses the effects of pamidronate in NHOK. (A) NHOK were treated with the indicated combinations (10 µM PAM, 1 µM FOH, 0.5 µM GGOH, 1 µM FOH/0.5 µM GGOH) for 6 days. Typical photographs were taken (100x). (B) NHOK were seeded at 2 × 104 cells per well and treated with the indicated combinations of PAM, FOH, GGOH, or FOH/GGOH at day 1. The numbers were counted after 3 and 6 days. The experiment was performed in triplicate, with bars representing standard errors. (C) NHOK were plated at 2 × 104 cells per well and treated with the indicated combinations of PAM, FOH, GGOH, or FOH/GGOH at day 1. After 6 days, cells were subjected to SA-β-gal activity. Cells in 3 independent fields were counted. Error bars represent standard errors.
Pamidronate Attenuates the Re-epithelialization of Oral Mucosal Cells
BPs have adverse effects on the proliferation of NHOK in the monolayer in vitro; however, their effects on a multi-layered oral mucosal epithelium are unknown. To examine effects of BPs at the tissue level, we utilized 3D organotypic oral mucosal tissue constructs, which recapitulated the connective tissue and differentiated epithelial tissue layers in vitro. H&E staining of the oral mucosal tissue constructs without any treatments revealed thickening of the epithelial layers that are similar to those of normal oral mucosal tissue (Fig. 4A, left panel). When PAM was used, thinner epithelial layers were evident (Fig. 4A, middle panel). More specifically, thinner basal and spinous layers of epithelium were observed in PAM-treated tissue constructs. In contrast, the cornified layers were intact, suggesting that PAM may expedite the epithelial differentiation process. IHC staining of PCNA showed reduced and weak positive staining in PAM-treated oral mucosal tissue constructs (Fig. 4A, bottom panels; Fig. 4C), indicating that PAM attenuated the proliferation of the oral epithelial cells. The oral mucosal tissue constructs treated with Lov, an inhibitor of the mevalonate pathway, showed similar results but to a greater extent (Fig. 4A, right panel). The re-epithelialization of the oral epithelial cells was also examined by the creation of a “wound”. In the control groups, oral epithelial cells migrated, repopulated, and differentiated to cover the wounded area (Fig. 4B, left panel). In contrast, PAM- and Lov-treated tissue constructs exhibited attenuated migration and proliferation in the wounded areas (Fig. 4B, middle and right panels), indicating that PAM impaired re-epithelialization of the oral mucosal cells. To confirm whether senescence was also induced in the PAM-treated oral mucosal constructs, we performed laser-captured microdissection (LCM) (Appendix Materials & Methods; Appendix Fig. 2) followed by qRT-PCR for senescence-associated genes such as IL-8 and MMP-3 (Kang et al., 2003; Freund et al., 2010). We found that the gene expression of IL-8 and MMP-3 was increased in the PAM- and Lov-treated oral mucosal tissue constructs (Figs. 4D,4E).
Figure 4.
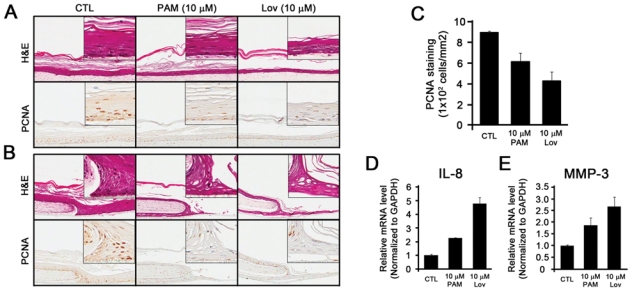
Pamidronate attenuates the re-epithelialization of oral mucosal cells. A 3D oral mucosal wound-healing model was established by the organotypic raft culture system. (A) Five days after air-lifting of the transwell, the oral mucosal tissue constructs were fed with medium only (CTL), medium containing 10 µM PAM, or medium containing 10 µM lovastatin (Lov). The oral tissue constructs were harvested 2 wks after the air-lifting, and subjected to H&E staining (top panel) and IHC staining against IgG (1:200, not shown) and PCNA (1:200) (bottom panel). (B) The circular wound was created 1 wk after the air-lifting. The oral tissue constructs were harvested after 7 days and subjected to H&E staining (top panel) and IHC staining against PCNA (bottom panel). (C) Three independent fields were randomly selected, and PCNA-positive cells were counted per mm2. The bar represents the standard deviation. (D, E) The epithelial cells of the oral mucosal tissue constructs were dissected by LCM. Total RNAs were isolated, cDNAs were synthesized, and qRT-PCR was performed for the expression of IL-8 (D) and MMP-3 (E). The experiments were performed in triplicate, and values were normalized to GAPDH.
Discussion
In this study, we report that BPs directly inhibit proliferation of NHOF and NHOK via two distinct mechanisms. More specifically, PAM asserts its anti-proliferative effects on NHOF mainly through apoptosis and NHOK through senescence. We also report that PAM impairs proliferation and migration, two processes important for the re-epithelialization of the oral mucosal cells. This was demonstrated by the wound-healing model in 3D oral mucosal tissue constructs, suggesting that oral mucosal cells may play an important role in the pathophysiology of BRONJ.
Previously, several groups studied direct effects of BPs on oral mucosal cells. They found that NHOF undergo apoptosis by BP (Scheper et al., 2009; Agis et al., 2010), which is consistent with our results (Fig. 1). However, the effects of BPs on oral keratinocytes remain controversial. For example, Scheper et al. showed apoptosis in keratinocytes by BPs, whereas Landesberg et al. (2008) observed retarded proliferation without apoptotic responses. However, a closer examination reveals that they utilized murine and immortalized keratinocytes, which behave differently from NHOK (Sanford and Evans, 1982; Chaturvedi et al., 1999). Using NHOK, we showed that PAM induced senescence, as demonstrated by enhanced SA-β-gal activity (Figs. 2D,2E), the increased molecular markers such as p16 and phosphorylated p38 (Figs. 2F,2G), and the increased expression of senescence-associated pro-inflammatory cytokines such as IL-6 and IL-8 (Figs. 2H,2I). Therefore, the effects of BPs seem to be cell-type-specific.
Senescent cells are physiologically relevant in vivo. Senescence is a tumor-suppressive mechanism to prevent transformation of precancerous cells (Adams, 2009). Senescent cells have been isolated from chronic non-healing wounds in humans (Mendez et al., 1998; Stanley and Osler, 2001). The inhibition of senescence mechanisms has been shown to accelerate wound closure (Jun and Lau, 2010), and mice exhibiting premature aging and senescence have been shown to have reduced re-epithelialization ability in wound-healing (Wang et al., 2007). Consistent with these findings, our wound-healing model in 3D oral mucosal tissue constructs showed impaired re-epithelialization (e.g., proliferation and migration) of oral mucosal cells (Figs. 4A,4B). Therefore, it is tempting to speculate that BP-induced senescence in oral keratinocytes mimics chronic non-healing wounds of the oral mucosal tissues by preventing and/or attenuating wound closure after dental trauma.
Recent clinical studies showed that the radiolabeled probes used in bone scintigraphy localize to the areas of the ONJ lesions (Zanglis et al., 2007; Dore et al., 2009; O’Ryan et al., 2009). The radiolabeled probes, 99mTc-labeled BP, utilize the bone-seeking property of BP which binds to the active turnover sites of the bone. Therefore, the uptake of 99mTc-labeled BP in the areas of ONJ lesions likely reflects the localization of BP. These findings suggest that the local concentration of BP in the areas of ONJ lesions may be comparable with, if not significantly higher than, those found in the serum. It is noteworthy that risk factors for BRONJ are tooth extraction and trauma under dental prosthesis, which are frequently accompanied by soft-tissue damage and the accumulation of blood at the local level. Interestingly, we found that NHOK underwent senescence at 10 µM, which is close to the upper therapeutic range of PAM serum concentration (0.73 – 2.61 µg/mL; US Package Insert, Novartis Pharmaceuticals). Therefore, senescence may be a physiologically relevant cellular response of NHOK in vivo in response to BPs.
The oral cavity is a unique and complex entity in the human body. Unlike other parts of the body, the oral mucosa is situated very close to the underlying bone. It is worth noting that: (1) there are minimal, if any, fat, fascia, or muscle tissues in these areas which can serve as a “cushion” or “insulator” between the oral mucosa and the BP-enriched underlying bone; and (2) BRONJ-prone areas exhibit thin oral mucosa, such as torus palatines and the crests of the maxilla/mandible. These anatomical factors are unique to the oral environment, may have the potential for exacerbation of the effects of bisphosphonates on the oral mucosal cells, and, therefore, may explain why BRONJ occurs in an oral-cavity-specific manner. Further in vivo studies will elucidate more precise roles of oral mucosa in BRONJ.
Supplementary Material
Acknowledgments
This study was supported by grants K08DE17121 and R03DE021114 (to R.H.K.) and R01DE18295 and K02DE18959 (to M.K.K.) from the National Institute of Dental and Craniofacial Research (NIDCR).
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Adams PD. (2009). Healing and hurting: molecular mechanisms, functions, and pathologies of cellular senescence. Mol Cell 36:2-14 [DOI] [PubMed] [Google Scholar]
- Adornato MC, Morcos I, Rozanski J. (2007). The treatment of BP associated osteonecrosis of the jaws with bone resection and autologous platelet-derived growth factors. J Am Dent Assoc 138:971-977 [DOI] [PubMed] [Google Scholar]
- Agis H, Blei J, Watzek G, Gruber R. (2010). Is zoledronate toxic to human periodontal fibroblasts? J Dent Res 89:40-45 [DOI] [PubMed] [Google Scholar]
- Chaturvedi V, Qin JZ, Denning MF, Choubey D, Diaz MO, Nickoloff BJ. (1999). Apoptosis in proliferating, senescent, and immortalized keratinocytes. J Biol Chem 274:23358-23367 [DOI] [PubMed] [Google Scholar]
- Chaturvedi V, Qin JZ, Stennett L, Choubey D, Nickoloff BJ. (2004). Resistance to UV-induced apoptosis in human keratinocytes during accelerated senescence is associated with functional inactivation of p53. J Cell Physiol 198:100-109 [DOI] [PubMed] [Google Scholar]
- Chen W, Dong Q, Shin KH, Kim RH, Oh JE, Park NH, et al. (2010). Grainyhead-like 2 enhances the human telomerase reverse transcriptase gene expression by inhibiting DNA methylation at the 5′-CpG island in normal human keratinocytes. J Biol Chem 285:40852-40863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groen PC, Lubbe DF, Hirsch LJ, Daifotis A, Stephenson W, Freedholm D, et al. (1996). Esophagitis associated with the use of alendronate. N Engl J Med 335:1016-2101 [DOI] [PubMed] [Google Scholar]
- Dore F, Filippi L, Biasotto M, Chiandussi S, Cavalli F, Di Lenarda R. (2009). Bone scintigraphy and SPECT/CT of bisphosphonate-induced osteonecrosis of the jaw. J Nucl Med 50:30-35 [DOI] [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez PY, Campisi J. (2010). Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med 16:238-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. (2010). The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12:676-685; erratum in Nat Cell Biol 12:1249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MK, Guo W, Park N-H. (1998). Replicative senescence of normal human oral keratinocytes is associated with the loss of telomerase activity without shortening of telomeres. Cell Growth Differ 9:85-95 [PubMed] [Google Scholar]
- Kang MK, Kameta A, Shin KH, Baluda MA, Kim HR, Park N-H. (2003). Senescence-associated genes in normal human oral keratinocytes. Exp Cell Res 287:272-281 [DOI] [PubMed] [Google Scholar]
- Kim RH, Lieberman MB, Lee R, Shin KH, Mehrazarin S, Oh JE, et al. (2010). Bmi-1 extends the life span of normal human oral keratinocytes by inhibiting the TGF-beta signaling. Exp Cell Res 316:2600-2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landesberg R, Cozin M, Cremers S, Woo V, Kousteni S, Sinha S, et al. (2008). Inhibition of oral mucosal cell wound healing by bisphosphonates. J Oral Maxillofac Surg 66:839-847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin L, Laviv A, Schwartz-Arad D. (2007). Denture-related osteonecrosis of the maxilla associated with oral bisphosphonate treatment. J Am Dent Assoc 138:1218-1220 [DOI] [PubMed] [Google Scholar]
- Marshall JK, Thabane M, James C. (2006). Randomized active and placebo-controlled endoscopy study of a novel protected formulation of oral alendronate. Dig Dis Sci 51:864-868 [DOI] [PubMed] [Google Scholar]
- Mendez MV, Stanley A, Park HY, Shon K, Phillips T, Menzoian JO. (1998). Fibroblasts cultured from venous ulcers display cellular characteristics of senescence. J Vasc Surg 28:876-883 [DOI] [PubMed] [Google Scholar]
- O’Ryan FS, Khoury S, Liao W, Han MM, Hui RL, Baer D, et al. (2009). Intravenous bisphosphonate-related osteonecrosis of the jaw: bone scintigraphy as an early indicator. J Oral Maxillofac Surg 67:1363-1372 [DOI] [PubMed] [Google Scholar]
- Qin JZ, Chaturvedi V, Denning MF, Bacon P, Panella J, Choubey D, et al. (2002). Regulation of apoptosis by p53 in UV-irradiated human epidermis, psoriatic plaques and senescent keratinocytes. Oncogene 21:2991-3002 [DOI] [PubMed] [Google Scholar]
- Reid IR, Bolland MJ, Grey AB. (2007). Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone 41:318-320 [DOI] [PubMed] [Google Scholar]
- Reszka AA, Rodan GA. (2004). Nitrogen-containing bisphosphonate mechanism of action. Mini Rev Med Chem 4:711-719 [PubMed] [Google Scholar]
- Rubegni P, Fimiani M. (2006). Images in clinical medicine. Bisphosphonate-associated contact stomatitis. N Engl J Med 355:e25. [DOI] [PubMed] [Google Scholar]
- Sanford KK, Evans VJ. (1982). A quest for the mechanism of ‘spontaneous’ malignant transformation in culture with associated advances in culture technology. J Natl Cancer Inst 68:895-913 [PubMed] [Google Scholar]
- Scheper MA, Badros A, Chaisuparat R, Cullen KJ, Meiller TF. (2009). Effect of zoledronic acid on oral fibroblasts and epithelial cells: a potential mechanism of bisphosphonate-associated osteonecrosis. Br J Haematol 144:667-676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin KH, Kang MK, Kim RH, Christensen R, Park NH. (2006). Heterogeneous nuclear ribonucleoprotein G shows tumor suppressive effect against oral squamous cell carcinoma cells. Clin Cancer Res 12:3222-3228 [DOI] [PubMed] [Google Scholar]
- Stanley A, Osler T. (2001). Senescence and the healing rates of venous ulcers. J Vasc Surg 33:1206-1211 [DOI] [PubMed] [Google Scholar]
- Van den Wyngaert T, Huizing MT, Vermorken JB. (2006). Bisphosphonates and osteonecrosis of the jaw: cause and effect or a post hoc fallacy? Ann Oncol 17:1197-1204 [DOI] [PubMed] [Google Scholar]
- Wang L, Yang L, Debidda M, Witte D, Zheng Y. (2007). Cdc42 GTPase-activating protein deficiency promotes genomic instability and premature aging-like phenotypes. Proc Natl Acad Sci USA 104:1248-1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts NB, Becker P. (1999). Alendronate increases spine and hip bone mineral density in women with postmenopausal osteoporosis who failed to respond to intermittent cyclical etidronate. Bone 24:65-68 [DOI] [PubMed] [Google Scholar]
- Zanglis A, Andreopoulos D, Dima M, Baltas G, Baziotis N. (2007). Jaw uptake of technetium-99 methylene diphosphonate in patients on biphosphonates: a word of caution. Hell J Nucl Med 10:177-180 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


