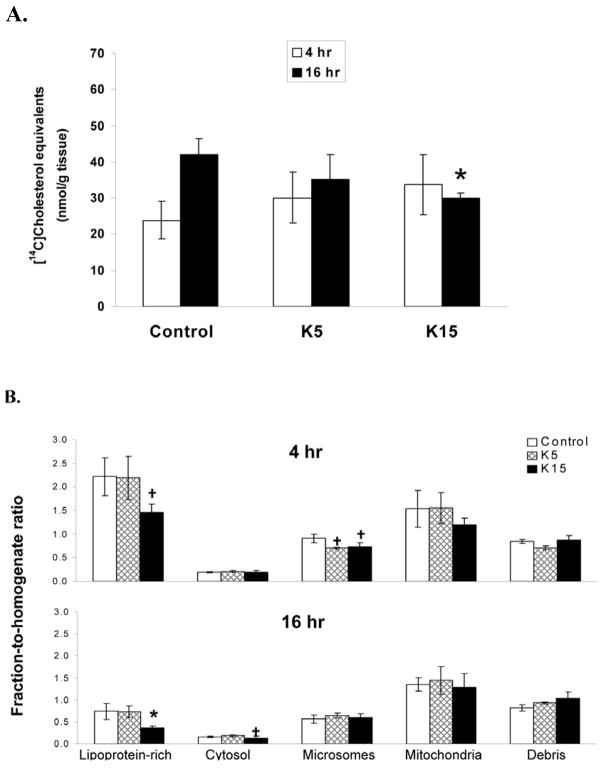
Figure 3. Hepatic disposition of [14C]CH equivalents of vehicle control and CD pretreated mice that received a challenge dose of [14C]CH.
Treatments were as described in Figure 1. A. Hepatic [14C]CH equivalents 4 and 16 h after ip bolus lipid of [14C]CH. B. Hepatic subcellular fraction to homogenate ratios. Hepatic subcellular fractions were prepared and analyzed from animals (6 mice in each group) as described under “material and methods”. Data are normalized by the amount of [14C]CH equivalents per mg protein in the liver homogenate. Values are expressed as mean ± SE. There were 6–7 mice in each group.*Indicates a statistically significant difference (p < 0.05) when compared with control. Indicates a statistically difference (p< 0.1) when compared with control.