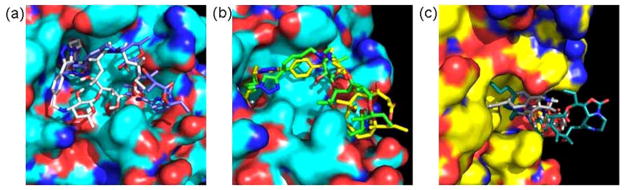
Fig. (9).
Docked structures of ketolide-derived HDACi at the active site of HDLP. (a) Superposition of the low energy conformation of 13b (grey) and 16b (blue) revealed the pocket binding preferences of inhibitors at the HDLP surface. (b) Relative orientation of the macrocyclic rings of 16a (yellow) and 16b (green) within the Phe 338 pocket. (c) Comparison of the orientation of the macrocyclic rings of the C6-linker compound 16b (grey) and C9-linker compound 16e (light-blue) within the Phe 338 pocket revealed the structural basis for the chain length dependence of the anti-HDAC activities of ketolide-derived HDACi.