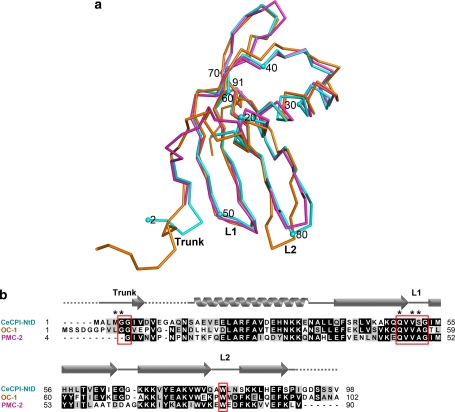
Fig. 3.
Structure comparison and sequence alignment of NtD of tarocystatin with OC-1 and PMC-2. a NtD of tarocystatin (CeCPI-NtD), OC-1, and PMC-2 are represented by cyan, orange, and magenta, respectively. The superimposition of the Cα atoms of NtD of tarocystatin, OC-1 and PMC2 gives an average RMS difference of 1.35 and 0.87 Å, respectively. The structure numbers indicate the residue number of NtD, and the conserved motifs are labeled by Trunk, L1 and L2. b The sequence alignment of CeCPI-NtD, OC-1, and PMC-2 reveals the similarity of the cystatin domain, except the loop of residues 10–15 of NtD is a highly flexible region. The residues of NtD interacting with papain by hydrogen bonding are labeled by asterisks. The secondary structure located above the sequence alignment is extracted from the structure of NtD, and the unresolved region is represented by dashed lines